Related Research Articles

Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown. In plants, these proteins are important for the biosynthesis of defensive compounds, fatty acids, and hormones.

Cytochrome P450 2A6 is a member of the cytochrome P450 mixed-function oxidase system, which is involved in the metabolism of xenobiotics in the body. CYP2A6 is the primary enzyme responsible for the oxidation of nicotine and cotinine. It is also involved in the metabolism of several pharmaceuticals, carcinogens, and a number of coumarin-type alkaloids. CYP2A6 is the only enzyme in the human body that appreciably catalyzes the 7-hydroxylation of coumarin, such that the formation of the product of this reaction, 7-hydroxycoumarin, is used as a probe for CYP2A6 activity.
Any enzyme system that includes cytochrome P450 protein or domain can be called a P450-containing system.
Cytochrome P450 1A2, a member of the cytochrome P450 mixed-function oxidase system, is involved in the metabolism of xenobiotics in the body. In humans, the CYP1A2 enzyme is encoded by the CYP1A2 gene.

Cytochrome P450 1B1 is an enzyme that in humans is encoded by the CYP1B1 gene.

Steroid 21-hydroxylase is an enzyme that hydroxylates steroids at the C21 position and is involved in biosynthesis of aldosterone and cortisol. The enzyme converts progesterone and 17α-hydroxyprogesterone into 11-deoxycorticosterone and 11-deoxycortisol, respectively, within metabolic pathways that ultimately lead to aldosterone and cortisol. Deficiency in the enzyme may cause congenital adrenal hyperplasia.

Cytochrome P450 3A5 is a protein that in humans is encoded by the CYP3A5 gene.

Cytochrome P450 2C18 is a protein that in humans is encoded by the CYP2C18 gene.

Cytochrome P450 2A13 is a protein that in humans is encoded by the CYP2A13 gene.

Cytochrome P450 3A43 is a protein that in humans is encoded by the CYP3A43 gene.

Cytochrome P450 2F1 is a protein that in humans is encoded by the CYP2F1 gene.

Cytochrome P450 4F8 is a protein that in humans is encoded by the CYP4F8 gene.

Cytochrome P450 4F12 is a protein that in humans is encoded by the CYP4F12 gene.

CYP4X1 is a protein which in humans is encoded by the CYP4X1 gene.

CYP2U1 is a protein that in humans is encoded by the CYP2U1 gene
Tyrosine N-monooxygenase (EC 1.14.13.41, tyrosine N-hydroxylase, CYP79A1) is an enzyme with systematic name L-tyrosine,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
Cholest-4-en-3-one 26-monooxygenase (EC 1.14.13.141, CYP125, CYP125A1, cholest-4-en-3-one 27-monooxygenase) is an enzyme with systematic name cholest-4-en-3-one,NADH:oxygen oxidoreductase (26-hydroxylating). This enzyme catalyses the following chemical reaction
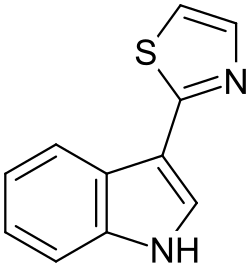
Camalexin (3-thiazol-2-yl-indole) is a simple indole alkaloid found in the plant Arabidopsis thaliana and other crucifers. The secondary metabolite functions as a phytoalexin to deter bacterial and fungal pathogens.
Cytochrome P450 omega hydroxylases, also termed cytochrome P450 ω-hydroxylases, CYP450 omega hydroxylases, CYP450 ω-hydroxylases, CYP omega hydroxylase, CYP ω-hydroxylases, fatty acid omega hydroxylases, cytochrome P450 monooxygenases, and fatty acid monooxygenases, are a set of cytochrome P450-containing enzymes that catalyze the addition of a hydroxyl residue to a fatty acid substrate. The CYP omega hydroxylases are often referred to as monoxygenases; however, the monooxygenases are CYP450 enzymes that add a hydroxyl group to a wide range of xenobiotic and naturally occurring endobiotic substrates, most of which are not fatty acids. The CYP450 omega hydroxylases are accordingly better viewed as a subset of monooxygenases that have the ability to hydroxylate fatty acids. While once regarded as functioning mainly in the catabolism of dietary fatty acids, the omega oxygenases are now considered critical in the production or break-down of fatty acid-derived mediators which are made by cells and act within their cells of origin as autocrine signaling agents or on nearby cells as paracrine signaling agents to regulate various functions such as blood pressure control and inflammation.
Cytochrome P450, family 107, also known as CYP107, is a cytochrome P450 monooxygenase family in bacteria, found to be conserved and highly populated in Streptomyces and Bacillus species. The first gene identified in this family is Cytochrome P450 eryF (CYP107A1) from Saccharopolyspora erythraea. Many enzymes of this family are involved in the synthesis of macrolide antibiotics. The members of this family are widely distributed in Alphaproteobacteria, cyanobacterial, Mycobacterium, Firmicutes and Streptomyces species, which may be due to horizontal gene transfer driven by selection pressure.
References
- ↑ Syed, PR; Chen, W; Nelson, DR; Kappo, AP; Yu, JH; Karpoormath, R; Syed, K (31 May 2019). "Cytochrome P450 Monooxygenase CYP139 Family Involved in the Synthesis of Secondary Metabolites in 824 Mycobacterial Species". International Journal of Molecular Sciences. 20 (11): 2690. doi: 10.3390/ijms20112690 . PMC 6600245 . PMID 31159249.