| Alpha-L-fucosidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
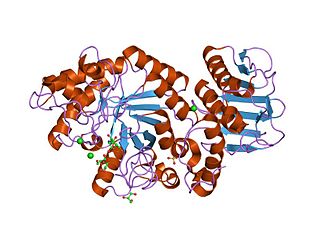
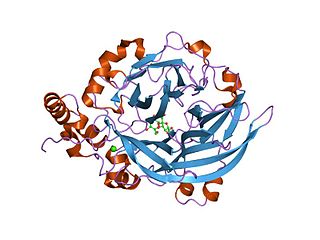
 crystal structure of thermotoga maritima alpha-fucosidase | |||||||||
| Identifiers | |||||||||
| Symbol | Alpha_L_fucos | ||||||||
| Pfam | PF01120 | ||||||||
| Pfam clan | CL0058 | ||||||||
| InterPro | IPR000933 | ||||||||
| PROSITE | PDOC00324 | ||||||||
| SCOP2 | 1hl9 / SCOPe / SUPFAM | ||||||||
| CAZy | GH29 | ||||||||
| |||||||||
In molecular biology, glycoside hydrolase family 29 is a family of glycoside hydrolases.
Glycoside hydrolases EC 3.2.1. are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycoside hydrolases, based on sequence similarity, has led to the definition of >100 different families. [1] [2] [3] This classification is available on the CAZy web site, [4] [5] and also discussed at CAZypedia, an online encyclopedia of carbohydrate active enzymes. [6] [7]
Glycoside hydrolase family 29 includes alpha-L-fucosidases, [8] They are lysosomal enzymes responsible for hydrolyzing the alpha-1,6-linked fucose joined to the reducing-end N-acetylglucosamine of the carbohydrate moieties of glycoproteins. Alpha-L-fucosidase is responsible for hydrolysing the alpha-1,6-linked fucose joined to the reducing-end N-acetylglucosamine of the carbohydrate moieties of glycoproteins.
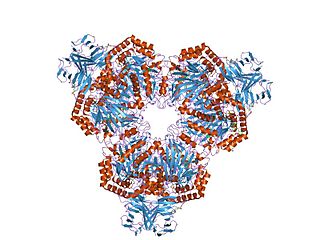
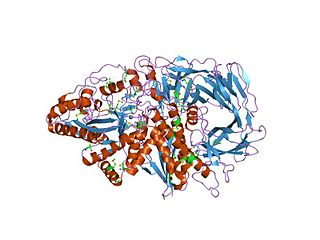
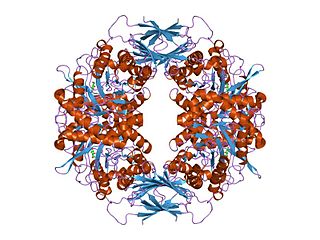
Fucosylated glycoconjugates are involved in numerous biological events, making alpha-l-fucosidases, the enzymes responsible for their processing, critically important. Deficiency in alpha-l-fucosidase activity is associated with fucosidosis, a lysosomal storage disorder characterised by rapid neurodegeneration, resulting in severe mental and motor deterioration. [9] The enzyme is a hexamer and displays a two-domain fold, composed of a catalytic (beta/alpha)(8)-like domain and a C-terminal beta-sandwich domain. [9]
Drosophila melanogaster spermatozoa contains an alpha-l-fucosidase that might be involved in fertilisation by interacting with alpha-l-fucose residues on the micropyle of the eggshell. [10] In human sperm, membrane-associated alpha-l-fucosidase is stable for extended periods of time, which is made possible by membrane domains and compartmentalisation. These help preserve protein integrity. [11]