
Infrared is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with waves that are just longer than those of red light, the longest waves in the visible spectrum, so IR is invisible to the human eye. IR is generally understood to include wavelengths from around 750 nm to 1 mm. IR is commonly divided between longer-wavelength thermal IR, emitted from terrestrial sources, and shorter-wavelength IR or near-IR, part of the solar spectrum. Longer IR wavelengths (30–100 μm) are sometimes included as part of the terahertz radiation band. Almost all black-body radiation from objects near room temperature is in the IR band. As a form of electromagnetic radiation, IR carries energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon.
In physiology, thermoception or thermoreception is the sensation and perception of temperature, or more accurately, temperature differences inferred from heat flux. It deals with a series of events and processes required for an organism to receive a temperature stimulus, convert it to a molecular signal, and recognize and characterize the signal in order to trigger an appropriate defense response.

Vampire bats, members of the subfamily Desmodontinae, are leaf-nosed bats currently found in Central and South America. Their food source is the blood of other animals, a dietary trait called hematophagy. Three extant bat species feed solely on blood: the common vampire bat, the hairy-legged vampire bat, and the white-winged vampire bat. Two extinct species of the genus Desmodus have been found in North America.

The New World leaf-nosed bats (Phyllostomidae) are bats found from southern North America to South America, specifically from the Southwest United States to northern Argentina. They are ecologically the most varied and diverse family within the order Chiroptera. Most species are insectivorous, but the phyllostomid bats include within their number true predatory species and frugivores. For example, the spectral bat, the largest bat in the Americas, eats vertebrate prey, including small, dove-sized birds. Members of this family have evolved to use food groups such as fruit, nectar, pollen, insects, frogs, other bats, and small vertebrates, and in the case of the vampire bats, even blood.

Desmodus is a genus of bats which—along with the genera Diaemus and Diphylla—are allied as the subfamily Desmodontinae, the carnivorous, blood-consuming vampire bats of the New World leaf-nosed bat family Phyllostomidae.
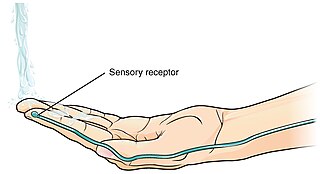
A thermoreceptor is a non-specialised sense receptor, or more accurately the receptive portion of a sensory neuron, that codes absolute and relative changes in temperature, primarily within the innocuous range. In the mammalian peripheral nervous system, warmth receptors are thought to be unmyelinated C-fibres, while those responding to cold have both C-fibers and thinly myelinated A delta fibers. The adequate stimulus for a warm receptor is warming, which results in an increase in their action potential discharge rate. Cooling results in a decrease in warm receptor discharge rate. For cold receptors their firing rate increases during cooling and decreases during warming. Some cold receptors also respond with a brief action potential discharge to high temperatures, i.e. typically above 45 °C, and this is known as a paradoxical response to heat. The mechanism responsible for this behavior has not been determined.

A nociceptor is a sensory neuron that responds to damaging or potentially damaging stimuli by sending "possible threat" signals to the spinal cord and the brain. The brain creates the sensation of pain to direct attention to the body part, so the threat can be mitigated; this process is called nociception.

The common vampire bat is a small, leaf-nosed bat native to the Neotropics. It is one of three extant species of vampire bat, the other two being the hairy-legged and the white-winged vampire bats. The common vampire bat practices hematophagy, mainly feeding on the blood of livestock. The bat usually approaches its prey at night while they are sleeping. It then uses its razor-sharp teeth to cut open the skin of its hosts and lap up their blood with its long tongue.
Chemesthesis is the detection of potentially harmful chemicals by the skin and mucous membranes. Chemesthetic sensations arise when chemical compounds activate receptors associated with other senses that mediate pain, touch, and thermal perception. These chemical-induced reactions do not fit into the traditional sense categories of taste and smell.

The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. Fatty acid metabolites with affinity for this receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA). The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception). In primary afferent sensory neurons, it cooperates with TRPA1 to mediate the detection of noxious environmental stimuli.
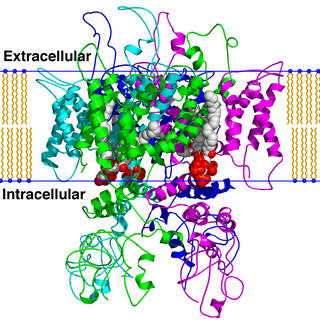
TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.

Capsazepine is a synthetic antagonist of capsaicin. It is used as a biochemical tool in the study of TRPV ion channels.

Transient receptor potential cation channel subfamily V member 2 is a protein that in humans is encoded by the TRPV2 gene. TRPV2 is a nonspecific cation channel that is a part of the TRP channel family. This channel allows the cell to communicate with its extracellular environment through the transfer of ions, and responds to noxious temperatures greater than 52 °C. It has a structure similar to that of potassium channels, and has similar functions throughout multiple species; recent research has also shown multiple interactions in the human body.

The ability to sense infrared thermal radiation evolved independently in two different groups of snakes, one consisting of the families Boidae (boas) and Pythonidae (pythons), the other of the family Crotalinae. What is commonly called a pit organ allows these animals to essentially "see" radiant heat at wavelengths between 5 and 30 μm. The more advanced infrared sense of pit vipers allows these animals to strike prey accurately even in the absence of light, and detect warm objects from several meters away. It was previously thought that the organs evolved primarily as prey detectors, but recent evidence suggests that it may also be used in thermoregulation and predator detection, making it a more general-purpose sensory organ than was supposed.
A sense is a biological system used by an organism for sensation, the process of gathering information about the surroundings through the detection of stimuli. Although, in some cultures, five human senses were traditionally identified as such, many more are now recognized. Senses used by non-human organisms are even greater in variety and number. During sensation, sense organs collect various stimuli for transduction, meaning transformation into a form that can be understood by the brain. Sensation and perception are fundamental to nearly every aspect of an organism's cognition, behavior and thought.
Relief from chronic pain remains a recognized unmet medical need. Consequently, the search for new analgesic agents is being intensively studied by the pharmaceutical industry. The TRPV1 receptor is a ligand gated ion channel that has been implicated in mediation of many types of pain and therefore studied most extensively. The first competitive antagonist, capsazepine, was first described in 1990; since then, several TRPV1 antagonists have entered clinical trials as analgesic agents. Should these new chemical entities relieve symptoms of chronic pain, then this class of compounds may offer one of the first novel mechanisms for the treatment of pain in many years.

David Jay Julius is an American physiologist and Nobel Prize laureate known for his work on molecular mechanisms of pain sensation and heat, including the characterization of the TRPV1 and TRPM8 receptors that detect capsaicin, menthol, and temperature. He is a professor at the University of California, San Francisco.
Zucapsaicin (Civanex) is a medication used to treat osteoarthritis of the knee and other neuropathic pain. Zucapsaicin is a member of phenols and a member of methoxybenzenes. It is a modulator of transient receptor potential cation channel subfamily V member 1 (TRPV-1), also known as the vanilloid or capsaicin receptor 1 that reduces pain, and improves articular functions. It is the cis-isomer of capsaicin. Civamide, manufactured by Winston Pharmaceuticals, is produced in formulations for oral, nasal, and topical use.

Vanillotoxins are neurotoxins found in the venom of the tarantula Psalmopoeus cambridgei. They act as agonists for the transient receptor potential cation channel subfamily V member 1 (TRPV1), activating the pain sensory system. VaTx1 and 2 also act as antagonists for the Kv2-type voltage-gated potassium channel (Kv2), inducing paralytic behavior in small animals.
RhTx is a small peptide toxin from Scolopendra subspinipes mutilans, also called the Chinese red-headed centipede. RhTx binds to the outer pore region of the temperature regulated TRPV1 ion channel, preferably in activated state, causing a downwards shift in the activation threshold temperature, which leads to the immediate onset of heat pain.















