A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, and enable mobility. Bones come in a variety of shapes and sizes and have complex internal and external structures. They are lightweight yet strong and hard and serve multiple functions.

Bone healing, or fracture healing, is a proliferative physiological process in which the body facilitates the repair of a bone fracture.
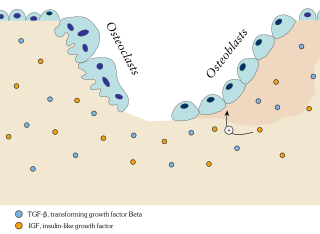
Osteoblasts are cells with a single nucleus that synthesize bone. However, in the process of bone formation, osteoblasts function in groups of connected cells. Individual cells cannot make bone. A group of organized osteoblasts together with the bone made by a unit of cells is usually called the osteon.

In histology, osteoid is the unmineralized, organic portion of the bone matrix that forms prior to the maturation of bone tissue. Osteoblasts begin the process of forming bone tissue by secreting the osteoid as several specific proteins. When it becomes mineralized, the osteoid and its adjacent bone cells have developed into new bone tissue.

An osteoclast is a type of bone cell that breaks down bone tissue. This function is critical in the maintenance, repair, and remodeling of bones of the vertebral skeleton. The osteoclast disassembles and digests the composite of hydrated protein and mineral at a molecular level by secreting acid and a collagenase, a process known as bone resorption. This process also helps regulate the level of blood calcium.

The periosteum is a membrane that covers the outer surface of all bones, except at the articular surfaces of long bones. Endosteum lines the inner surface of the medullary cavity of all long bones.

An osteocyte, an oblate shaped type of bone cell with dendritic processes, is the most commonly found cell in mature bone. It can live as long as the organism itself. The adult human body has about 42 billion of them. Osteocytes do not divide and have an average half life of 25 years. They are derived from osteoprogenitor cells, some of which differentiate into active osteoblasts. Osteoblasts/osteocytes develop in mesenchyme.

Chondrocytes are the only cells found in healthy cartilage. They produce and maintain the cartilaginous matrix, which consists mainly of collagen and proteoglycans. Although the word chondroblast is commonly used to describe an immature chondrocyte, the term is imprecise, since the progenitor of chondrocytes can differentiate into various cell types, including osteoblasts.
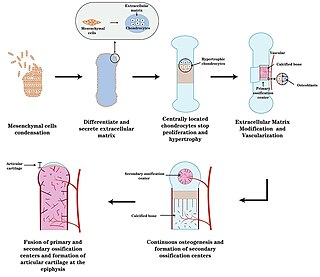
Endochondral ossification is one of the two essential pathways by which bone tissue is produced during fetal development of the mammalian skeletal system, the other pathway being intramembranous ossification. Both endochondral and intramembranous processes initiate from a precursor mesenchymal tissue, but their transformations into bone are different. In intramembranous ossification, mesenchymal tissue is directly converted into bone. On the other hand, endochondral ossification starts with mesenchymal tissue turning into an intermediate cartilage stage, which is eventually substituted by bone.

In osteology, the osteon or haversian system is the fundamental functional unit of much compact bone. Osteons are roughly cylindrical structures that are typically between 0.25 mm and 0.35 mm in diameter. Their length is often hard to define, but estimates vary from several millimeters to around 1 centimeter. They are present in many bones of most mammals and some bird, reptile, and amphibian species.
Ossification in bone remodeling is the process of laying down new bone material by cells named osteoblasts. It is synonymous with bone tissue formation. There are two processes resulting in the formation of normal, healthy bone tissue: Intramembranous ossification is the direct laying down of bone into the primitive connective tissue (mesenchyme), while endochondral ossification involves cartilage as a precursor.

Bone resorption is resorption of bone tissue, that is, the process by which osteoclasts break down the tissue in bones and release the minerals, resulting in a transfer of calcium from bone tissue to the blood.

Chondroblasts, or perichondrial cells, is the name given to mesenchymal progenitor cells in situ which, from endochondral ossification, will form chondrocytes in the growing cartilage matrix. Another name for them is subchondral cortico-spongious progenitors. They have euchromatic nuclei and stain by basic dyes.

Osteonecrosis of the jaw (ONJ) is a severe bone disease (osteonecrosis) that affects the jaws. Various forms of ONJ have been described since 1861, and a number of causes have been suggested in the literature.

In osteology, bone remodeling or bone metabolism is a lifelong process where mature bone tissue is removed from the skeleton and new bone tissue is formed. These processes also control the reshaping or replacement of bone following injuries like fractures but also micro-damage, which occurs during normal activity. Remodeling responds also to functional demands of the mechanical loading.

Transcription factor Sp7, also called osterix (Osx), is a protein that in humans is encoded by the SP7 gene. It is a member of the Sp family of zinc-finger transcription factors It is highly conserved among bone-forming vertebrate species It plays a major role, along with Runx2 and Dlx5 in driving the differentiation of mesenchymal precursor cells into osteoblasts and eventually osteocytes. Sp7 also plays a regulatory role by inhibiting chondrocyte differentiation maintaining the balance between differentiation of mesenchymal precursor cells into ossified bone or cartilage. Mutations of this gene have been associated with multiple dysfunctional bone phenotypes in vertebrates. During development, a mouse embryo model with Sp7 expression knocked out had no formation of bone tissue. Through the use of GWAS studies, the Sp7 locus in humans has been strongly associated with bone mass density. In addition there is significant genetic evidence for its role in diseases such as Osteogenesis imperfecta (OI).

Osteochondroprogenitor cells are progenitor cells that arise from mesenchymal stem cells (MSC) in the bone marrow. They have the ability to differentiate into osteoblasts or chondrocytes depending on the signalling molecules they are exposed to, giving rise to either bone or cartilage respectively. Osteochondroprogenitor cells are important for bone formation and maintenance.
Wolff's law, developed by the German anatomist and surgeon Julius Wolff (1836–1902) in the 19th century, states that bone in a healthy animal will adapt to the loads under which it is placed. If loading on a particular bone increases, the bone will remodel itself over time to become stronger to resist that sort of loading. The internal architecture of the trabeculae undergoes adaptive changes, followed by secondary changes to the external cortical portion of the bone, perhaps becoming thicker as a result. The inverse is true as well: if the loading on a bone decreases, the bone will become less dense and weaker due to the lack of the stimulus required for continued remodeling. This reduction in bone density (osteopenia) is known as stress shielding and can occur as a result of a hip replacement. The normal stress on a bone is shielded from that bone by being placed on a prosthetic implant.

Mesenchymal stem cells (MSCs) also known as mesenchymal stromal cells or medicinal signaling cells, are multipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts, chondrocytes, myocytes and adipocytes.
Craniofacial regeneration refers to the biological process by which the skull and face regrow to heal an injury. This page covers birth defects and injuries related to the craniofacial region, the mechanisms behind the regeneration, the medical application of these processes, and the scientific research conducted on this specific regeneration. This regeneration is not to be confused with tooth regeneration. Craniofacial regrowth is broadly related to the mechanisms of general bone healing.