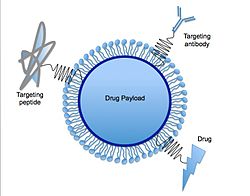
Drug delivery
Solid lipid nanoparticles can function as the basis for oral and parenteral drug delivery systems. SLNs combine the advantages of lipid emulsion and polymeric nanoparticle systems while overcoming the temporal and in vivo stability issues that troubles the conventional as well as polymeric nanoparticles drug delivery approaches. [10] It has been proposed that SLNs have many advantages over other colloidal carriers i.e. incorporation of lipophilic and hydrophilic drugs feasible, no biotoxicity of the carrier, avoidance of organic solvents, possibility of controlled drug release and drug targeting, increased drug stability and no problems with respect to large scale production. [10] Various functions such as molecules for targeting, PEG chains for stealth properties, [18] or thiol groups for adhesion via disulfide bond formation [19] can be immobilized on their surface. A recent study has demonstrated the use of solid lipid nanoparticles as a platform for oral delivery of the nutrient mineral iron, by incorporating the hydrophilic molecule ferrous sulfate (FeSO4) in a lipid matrix composed of stearic acid. [20] Carvedilol-loaded solid lipid nanoparticles were prepared using hot-homogenization technique for oral delivery with compritol and poloxamer 188 as the lipid and surfactant, respectively. [21] Another example of drug delivery using SLN would be oral solid SLN suspended in distilled water, which was synthesized to trap drugs within the SLN structure. Upon ingestion, the SLNs are exposed to gastric and intestinal acids that dissolve the SLNs and release the drugs into the system. [22]
Many nano-structured systems have been employed for ocular drug delivery. SLNs have been looked at as a potential drug carrier system since the 1990s. SLNs do not show biotoxicity as they are prepared from physiological lipids. SLNs are useful in ocular drug delivery as they can enhance the corneal absorption of drugs and improve the ocular bioavailability of both hydrophilic and lipophilic drugs. [23] SLNs have another advantage of allowing autoclave sterilization, a necessary step towards formulation of ocular preparations. [24]
Advantages of SLNs include the use of physiological lipids (which decreases the danger of acute and chronic toxicity), the avoidance of organic solvents, a potential wide application spectrum (dermal, per os, intravenous) and the high pressure homogenization as an established production method. Additionally, improved bioavailability, protection of sensitive drug molecules from the outer environment (e.g. water, light), and even controlled release characteristics were claimed by the incorporation of poorly water-soluble drugs in the solid lipid matrix. Moreover, SLNs can carry both lipophilic and hydrophilic drugs, and are more affordable compared to polymeric/surfactant-based carriers. [25]
Nucleic acids
A significant obstacle to using LNPs as a delivery vehicle for nucleic acids is that in nature, lipids and nucleic acids both carry a negative electric charge—meaning they do not easily mix with each other. [26] While working at Syntex in the mid-1980s, [27] Philip Felgner pioneered the use of artificially-created cationic lipids (positively-charged lipids) to bind lipids to nucleic acids in order to transfect the latter into cells. [28] However, by the late 1990s, it was known from in vitro experiments that this use of cationic lipids had undesired side effects on cell membranes. [29]
During the late 1990s and 2000s, Pieter Cullis, while at the University of British Columbia, developed ionizable cationic lipids which are "positively charged at an acidic pH but neutral in the blood." [6] Cullis also led the development of a technique involving careful adjustments to pH during the process of mixing ingredients in order to create LNPs which could safely pass through the cell membranes of living organisms. [26] [30] As of 2021, the current understanding of LNPs formulated with such ionizable cationic lipids is that they enter cells through receptor-mediated endocytosis and end up inside endosomes. [6] The acidity inside the endosomes causes LNPs' ionizable cationic lipids to acquire a positive charge, and this is thought to allow LNPs to escape from endosomes and release their RNA payloads. [6]
From 2005 into the early 2010s, LNPs were investigated as a drug delivery system for small interfering RNA (siRNA) drugs. [6] In 2009, Cullis co-founded a company called Acuitas Therapeutics to commercialize his LNP research; Acuitas worked on developing LNPs for Alnylam Pharmaceuticals's siRNA drugs. [31] In 2018, the FDA approved Alnylam's siRNA drug Onpattro (patisiran), the first drug to use LNPs as the drug delivery system. [4] [6]
By that point in time, siRNA drug developers like Alnylam were already looking at other options for future drugs like chemical conjugate systems, but during the 2010s, the earlier research into using LNPs for siRNA became a foundation for new research into using LNPs for mRNA. [6] Lipids intended for short siRNA strands did not work well for much longer mRNA strands, which led to extensive research during the mid-2010s into the creation of novel ionizable cationic lipids appropriate for mRNA. [6] As of late 2020, several mRNA vaccines for SARS-CoV-2 use LNPs as their drug delivery system, including both the Moderna COVID-19 vaccine and the Pfizer–BioNTech COVID-19 vaccines. [4] Moderna uses its own proprietary ionizable cationic lipid called SM-102, while Pfizer and BioNTech licensed an ionizable cationic lipid called ALC-0315 from Acuitas. [6]