
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle.

Methionine synthase also known as MS, MeSe, MTR is responsible for the regeneration of methionine from homocysteine. In humans it is encoded by the MTR gene (5-methyltetrahydrofolate-homocysteine methyltransferase). Methionine synthase forms part of the S-adenosylmethionine (SAMe) biosynthesis and regeneration cycle, and is the enzyme responsible for linking the cycle to one-carbon metabolism via the folate cycle. There are two primary forms of this enzyme, the Vitamin B12 (cobalamin)-dependent (MetH) and independent (MetE) forms, although minimal core methionine synthases that do not fit cleanly into either category have also been described in some anaerobic bacteria. The two dominant forms of the enzymes appear to be evolutionary independent and rely on considerably different chemical mechanisms. Mammals and other higher eukaryotes express only the cobalamin-dependent form. In contrast, the distribution of the two forms in Archaeplastida (plants and algae) is more complex. Plants exclusively possess the cobalamin-independent form, while algae have either one of the two, depending on species. Many different microorganisms express both the cobalamin-dependent and cobalamin-independent forms.
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is converted into fatty acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway also provides the glycerol with which three fatty acids can combine to form triglycerides, the final product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol group is phosphorylated with a group such as phosphatidylcholine, a phospholipid is formed. Phospholipids form the bulk of the lipid bilayers that make up cell membranes and surrounds the organelles within the cells.

Cystathionine-β-synthase, also known as CBS, is an enzyme (EC 4.2.1.22) that in humans is encoded by the CBS gene. It catalyzes the first step of the transsulfuration pathway, from homocysteine to cystathionine:
In enzymology, a 7-methylxanthosine synthase is an enzyme that catalyzes the chemical reaction
In enzymology, a corydaline synthase (EC 2.1.1.147) is an enzyme that catalyzes the chemical reaction
In enzymology, a cyclopropane-fatty-acyl-phospholipid synthase is an enzyme that catalyzes the chemical reaction
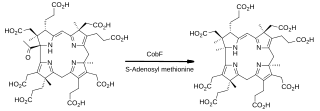
In enzymology, precorrin-6A synthase (deacetylating) (EC 2.1.1.152) is an enzyme that catalyzes the chemical reaction
In enzymology, a theobromine synthase is an enzyme that catalyzes the chemical reaction

[Methionine synthase] reductase, or Methionine synthase reductase, encoded by the gene MTRR, is an enzyme that is responsible for the reduction of methionine synthase inside human body. This enzyme is crucial for maintaining the one carbon metabolism, specifically the folate cycle. The enzyme employs one coenzyme, flavoprotein.
In enzymology, a 2-ethylmalate synthase (EC 2.3.3.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a 6'-deoxychalcone synthase (EC 2.3.1.170) is an enzyme that catalyzes the chemical reaction
In enzymology, a 6-methylsalicylic-acid synthase (EC 2.3.1.165) is a polyketide synthase that catalyzes the chemical reaction

Fatty-acyl-CoA Synthase, or more commonly known as yeast fatty acid synthase, is an enzyme complex responsible for fatty acid biosynthesis, and is of Type I Fatty Acid Synthesis (FAS). Yeast fatty acid synthase plays a pivotal role in fatty acid synthesis. It is a 2.6 MDa barrel shaped complex and is composed of two, unique multi-functional subunits: alpha and beta. Together, the alpha and beta units are arranged in an α6β6 structure. The catalytic activities of this enzyme complex involves a coordination system of enzymatic reactions between the alpha and beta subunits. The enzyme complex therefore consists of six functional centers for fatty acid synthesis.
In enzymology, an icosanoyl-CoA synthase (EC 2.3.1.119) is an enzyme that catalyzes the chemical reaction
In enzymology, a mycocerosate synthase (EC 2.3.1.111) is an enzyme that catalyzes the chemical reaction
In enzymology, a trihydroxystilbene synthase (EC 2.3.1.95) is an enzyme that catalyzes the chemical reaction

Methionine synthase reductase, also known as MSR, is an enzyme that in humans is encoded by the MTRR gene.

Cobalamin biosynthesis is the process by which bacteria and archea make cobalamin, vitamin B12. Many steps are involved in converting aminolevulinic acid via uroporphyrinogen III and adenosylcobyric acid to the final forms in which it is used by enzymes in both the producing organisms and other species, including humans who acquire it through their diet.

Chlorophyllide a and Chlorophyllide b are the biosynthetic precursors of chlorophyll a and chlorophyll b respectively. Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway. Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria. Chlorophyllide a is also an intermediate in the biosynthesis of bacteriochlorophylls.









