Interactions
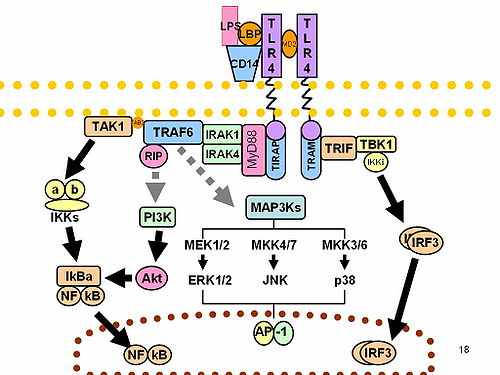
Lymphocyte antigen 96 has been shown to interact with TLR 4. [5] [11]
When LPS binds to a hydrophobic pocket in MD-2, it directly mediates dimerization of the two TLR4-MD-2 complexes. Thus, TLR4 and MD-2 form a heterodimer that recognizes a common pattern in structurally diverse LPS molecules. These interactions allow TLR4 to recognize LPS. [8] Macrophages in MD-2 knockout mice are unresponsive to LPS. [12]
LPS is extracted from the bacterial membrane and transferred to TLR4-MD-2 by two accessory proteins, LPS-binding protein and CD14, to induce innate immune response. [8]
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.