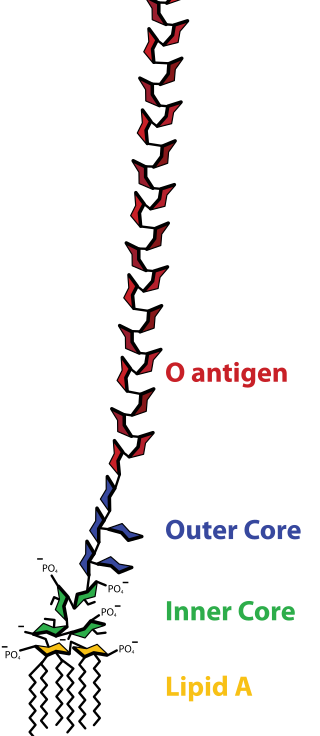
Lipopolysaccharides (LPS) are large molecules consisting of a lipid and a polysaccharide that are bacterial toxins. They are composed of an O-antigen, an outer core, and an inner core all joined by covalent bonds, and are found in the bacterial capsule, the outermost membrane of cell envelope of Gram-negative bacteria, such as E. coli and Salmonella. Today, the term endotoxin is often used synonymously with LPS, although there are a few endotoxins that are not related to LPS, such as the so-called delta endotoxin proteins produced by Bacillus thuringiensis.

Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-spanning receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize structurally conserved molecules derived from microbes. Once these microbes have reached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13. Humans lack genes for TLR11, TLR12 and TLR13 and mice lack a functional gene for TLR10. The receptors TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in intracellular vesicles.

CD14 is a human protein made mostly by macrophages as part of the innate immune system. It helps to detect bacteria in the body by binding lipopolysaccharide (LPS), a pathogen-associated molecular pattern (PAMP).

Myeloid differentiation primary response 88 (MYD88) is a protein that, in humans, is encoded by the MYD88 gene.
Bactericidal permeability-increasing protein (BPI) is a 456-residue (~50kDa) protein that is part of the innate immune system, coded for in the human by the BPI gene. It belongs to the family of lipid-binding serum glycoproteins.

BPI fold containing family A, member 1 (BPIFA1), also known as Palate, lung, and nasal epithelium clone (PLUNC), is a protein that in humans is encoded by the BPIFA1 gene. It was also formerly known as "Secretory protein in upper respiratory tracts" (SPURT). The BPIFA1 gene sequence predicts 4 transcripts ; 3 mRNA variants have been well characterized. The resulting BPIFA1 is a secreted protein, expressed at very high levels in mucosa of the airways and salivary glands; at high levels in oropharyneal epithelium, including tongue and tonsils; and at moderate levels many other tissue types and glands including pituitary, testis, lung, bladder, blood, prostate, pancreas, levels in the digestive tract and pancreas. The protein can be detected on the apical side of epithelial cells and in airway surface liquid, nasal mucus, and sputum.

Toll-like receptor 4 is a protein that in humans is encoded by the TLR4 gene. TLR4 is a transmembrane protein, member of the toll-like receptor family, which belongs to the pattern recognition receptor (PRR) family. Its activation leads to an intracellular signaling pathway NF-κB and inflammatory cytokine production which is responsible for activating the innate immune system.

Lymphocyte antigen 96, also known as "Myeloid Differentiation factor 2 (MD-2)," is a protein that in humans is encoded by the LY96 gene.

BPI fold-containing family B, member 2, (BPIFB2) also known as bactericidal/permeability-increasing protein-like 1, is a protein that in humans is encoded by the BPIFB2 gene.

Acyloxyacyl hydrolase, also known as AOAH, is a eukaryotic protein encoded by the AOAH gene. AOAH is produced by macrophages, dendritic cells, NK cells, ILC1 cells, neutrophils and renal proximal tubule cells.
In molecular biology, the lipid-binding serum glycoproteins family, also known as the BPI/LBP/Plunc family or LBP/BPI/CETP family represents a family which includes mammalian lipid-binding serum glycoproteins and/or proteins containing a structural motif known as the BPI fold. Members of this family include:

BPI fold containing family A, member 3 (BPIFA3) is a protein that in humans is encoded by the BPIFA3 gene. The gene is also known as SPLUNC3 and C20orf71 in humans and the orthologous gene in mice is 1700058C13Rik. There are multiple variants of the BPIFA3 projected to be a secreted protein. It is very highly expressed in testis with little or no expression in other tissues. The Human Protein Atlas project and Mouse ENCODE Consortium report RNA-Seq expression at RPKM levels of 29.1 for human testis and 69.4 for mouse, but 0 for all other tissues. Similarly, the Bgee consortium, using multiple techniques in addition to RNA-Seq, reports a relative Expression Score of 95.8 out of 100 for testis and 99.0 for sperm in humans; however low levels of BPIFA3 between 20 and 30 were seen for a variety of tissues such as muscle, glands, prostate, nervous system, and skin.

BPI fold containing family B, member 4 (BPIFB4) is a protein that in humans is encoded by the BPIFB4 gene. It was formerly known as "Long palate, lung and nasal epithelium carcinoma-associated protein 4" encoded by the LPLUNC4 gene. The BPIFB4 gene sequence predicts 4 transcripts ; 3 isoforms have been well characterized. In a variety of mammals, BPIFB4 is generally expressed in very high levels in the olfactory epithelium, high levels in the gonads and pituitary, moderate levels in white blood cells (monocytes) It can occur either localized in the cytoplasm of cells or secreted and circulated systemically in blood plasma.

BPI fold-containing family B member 1 (BPIFB1) is a protein that in humans is encoded by the BPIFB1 gene. BPIFB1 is a secreted protein, expressed at very high levels in mucosa of the airways and salivary glands, and at moderate levels in the digestive tract and pancreas.

BPI fold containing family B, member 3 (BPIFB3) is a protein that in humans is encoded by the BPIFB3 gene. Two variants have been detected in humans.

BPI fold containing family B, member 5 is a non-human protein encoded by the Bpifb5 gene, also known as Lplunc5. The BPIFB5 protein and Bpifb5 gene have been characterized in mammals such as rodents and even-toed ungulates but are apparently lacking in primates and other vertebrates such as birds, reptiles, and amphibians. The protein in rodents is expressed at moderately high levels in mucosa of the airways and at moderate levels in salivary glands, esophagus, and gonads ; in even-toed ungulates expression is high in testis, moderate in brain and striated muscle, and low in kidney.

BPI fold containing family B, member 6 (BPIFB6), also known as bactericidal/permeability-increasing protein-like 3 (BPIL3), is a protein that in humans is encoded by the BPIFB6 gene, also known as BPIL3 and LPLUNC6. It is expressed at high levels in hypertrophic tonsils, at relatively moderate levels in oronasal epithelium including nasal mucosa, tongue, and salivary gland, as well as esophageal mucosa at lesser levels. Orthologs are present in many vertebrate species including mammals, birds, reptiles, and amphibians.

Vomeromodulin is a non-human protein also known as BPI fold containing family B, member 9 (BPIFB9) in the rat encoded by the Bpifb9/RYF3 gene, and as BPI fold containing family B, member 9A (BPIFB9A) encoded by the Bpifb9a gene in the mouse. This protein has been characterized in mammals such as rodents, carnivores, even-toed ungulates, insectivores, bats, lagomorphs, and shrews but is apparently absent in primates and other vertebrates such as birds, reptiles, and amphibians. Its function is associated with detection of chemical odorant pheromone molecules.

BPI fold containing family A, member 2 (BPIFA2), also known as Parotid Secretory Protein (PSP), is a protein that in humans is encoded by the BPIFA2 gene. The BPIFA2 gene sequence predicts multiple transcripts ; 2 mRNA variants have been well characterized. The resulting BPIFA2 is a secreted protein, expressed at very high levels in the parotid (salivary) gland; at high levels in oropharyngeal mucosa, including tongue; and at moderate levels many other tissue types and glands including mammary gland, testis, lung, bladder, blood, prostate, adrenal gland, kidney, and pancreas.

BPI fold containing family A, member 4 (BPIFA4) is a non-human protein encoded by the Bpifa4 gene in mammals such as monkey, cat, and cow but does not appear in rodents and humans. It is also known as Latherin in horse, encoded by the Lath/Bpifa4 gene but is somewhat divergent from the other species. Latherin/BPIFA4 is a secreted protein found in saliva and sweat.