
Carotenoids are yellow, orange, and red organic pigments that are produced by plants and algae, as well as several bacteria, archaea, and fungi. Carotenoids give the characteristic color to pumpkins, carrots, parsnips, corn, tomatoes, canaries, flamingos, salmon, lobster, shrimp, and daffodils. Over 1,100 identified carotenoids can be further categorized into two classes – xanthophylls and carotenes.

Xanthophylls are yellow pigments that occur widely in nature and form one of two major divisions of the carotenoid group; the other division is formed by the carotenes. The name is from Greek: xanthos (ξανθός), meaning "yellow", and phyllon (φύλλον), meaning "leaf"), due to their formation of the yellow band seen in early chromatography of leaf pigments.

Carotenoid oxygenases are a family of enzymes involved in the cleavage of carotenoids to produce, for example, retinol, commonly known as vitamin A. This family includes an enzyme known as RPE65 which is abundantly expressed in the retinal pigment epithelium where it catalyzed the formation of 11-cis-retinol from all-trans-retinyl esters.

Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides. It is a yellow crystalline solid that has been used to dye wool.

Daidzein is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones are produced in plants through the phenylpropanoid pathway of secondary metabolism and are used as signal carriers, and defense responses to pathogenic attacks. In humans, recent research has shown the viability of using daidzein in medicine for menopausal relief, osteoporosis, blood cholesterol, and lowering the risk of some hormone-related cancers, and heart disease. Despite the known health benefits, the use of both puerarin and daidzein is limited by their poor bioavailability and low water solubility.
CRT is the gene cluster responsible for the biosynthesis of carotenoids. Those genes are found in eubacteria, in algae and are cryptic in Streptomyces griseus.

Prostaglandin-I synthase also known as prostaglandin I2 (prostacyclin) synthase (PTGIS) or CYP8A1 is an enzyme involved in prostanoid biosynthesis that in humans is encoded by the PTGIS gene. This enzyme belongs to the family of cytochrome P450 isomerases.
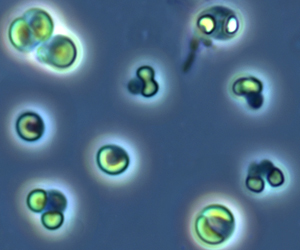
Picocystis is a monotypic genus of green algae, the sole species is Picocystis salinarum. It is placed within its own class, Picocystophyceae in the division Chlorophyta.
In enzymology, a copalyl diphosphate synthase is an enzyme that catalyzes the chemical reaction
In enzymology, an ent-copalyl diphosphate synthase is an enzyme that catalyzes the chemical reaction:
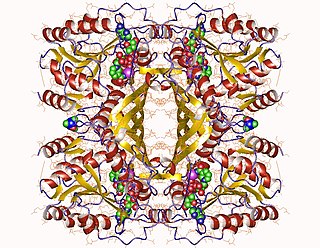
In enzymology, an inositol-3-phosphate synthase is an enzyme that catalyzes the chemical reaction

In enzymology, a prostaglandin-D synthase is an enzyme that catalyzes the chemical reaction

Damascenones are a series of closely related chemical compounds that are components of a variety of essential oils. The damascenones belong to a family of chemicals known as rose ketones, which also includes damascones and ionones. beta-Damascenone is a major contributor to the aroma of roses, despite its very low concentration, and is an important fragrance chemical used in perfumery.
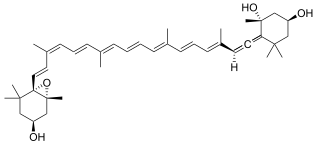
Neoxanthin is a carotenoid and xanthophyll. In plants, it is an intermediate in the biosynthesis of the plant hormone abscisic acid. It is often present in two forms: all-trans and 9-cis isomers. It is produced from violaxanthin, but a suspected neoxanthin synthase is still to be confirmed. Two different genes were confirmed to be implied in violaxanthin conversion to neoxanthin in Arabidopsis and tomato. It has a specific role in protection against photooxidative stress. It is a major xanthophyll found in green leafy vegetables such as spinach.

15-cis-phytoene desaturases, are enzymes involved in the carotenoid biosynthesis in plants and cyanobacteria. Phytoene desaturases are membrane-bound enzymes localized in plastids and introduce two double bonds into their colorless substrate phytoene by dehydrogenation and isomerize two additional double bonds. This reaction starts a biochemical pathway involving three further enzymes called the poly-cis pathway and leads to the red colored lycopene. The homologous phytoene desaturase found in bacteria and fungi (CrtI) converts phytoene directly to lycopene by an all-trans pathway.

Phytoene desaturase (lycopene-forming) are enzymes found in archaea, bacteria and fungi that are involved in carotenoid biosynthesis. They catalyze the conversion of colorless 15-cis-phytoene into a bright red lycopene in a biochemical pathway called the poly-trans pathway. The same process in plants and cyanobacteria utilizes four separate enzymes in a poly-cis pathway.
Carlactone synthase (EC 1.13.11.69, CCD8 (gene), MAX4 (gene), NCED8 (gene)) is an enzyme with systematic name 9-cis-10'-apo-beta-carotenal:O2 oxidoreductase (14,15-cleaving, carlactone-forming). This enzyme catalyses the following chemical reaction
Prolycopene isomerase is an enzyme with systematic name 7,9,7',9'-tetracis-lycopene cis-trans-isomerase. This enzyme catalyses the following chemical reaction
Capsanthin/capsorubin synthase is an enzyme with systematic name violaxanthin—capsorubin isomerase (ketone-forming). This enzyme catalyses the following chemical reaction
9,12-octadecadienoate 8-hydroperoxide 8R-isomerase is an enzyme with systematic name (8R,9Z,12Z)-8-hydroperoxyoctadeca-9,12-dienoate hydroxymutase ( -5,8-dihydroxyoctadeca-9,12-dienoate-forming). This enzyme catalyses the following chemical reaction













