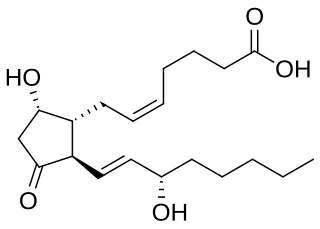
Prostaglandin D2 (or PGD2) is a prostaglandin that binds to the receptor PTGDR (DP1), as well as CRTH2 (DP2). It is a major prostaglandin produced by mast cells – recruits Th2 cells, eosinophils, and basophils. In mammalian organs, large amounts of PGD2 are found only in the brain and in mast cells. It is critical to development of allergic diseases such as asthma. Research carried out in 1989 found PGD2 is the primary mediator of vasodilation (the "niacin flush") after ingestion of niacin (nicotinic acid).

Prostaglandin-I synthase also known as prostaglandin I2 (prostacyclin) synthase (PTGIS) or CYP8A1 is an enzyme involved in prostanoid biosynthesis that in humans is encoded by the PTGIS gene. This enzyme belongs to the family of cytochrome P450 isomerases.

Prostaglandin-H2 D-isomerase (PTGDS) is an enzyme that in humans is encoded by the PTGDS gene.
In enzymology, a prostaglandin-E2 9-reductase (EC 1.1.1.189) is an enzyme that catalyzes the chemical reaction
In enzymology, a 15-hydroxyprostaglandin-D dehydrogenase (NADP+) (EC 1.1.1.196) is an enzyme that catalyzes the chemical reaction

Hydroxyprostaglandin dehydrogenase 15-(NAD) (the HUGO-approved symbol = HPGD; HGNC ID, HGNC:5154), also called 15-hydroxyprostaglandin dehydrogenase (NAD+), (EC 1.1.1.141), is an enzyme that catalyzes the following chemical reaction:
In enzymology, a 15-hydroxyprostaglandin dehydrogenase (NADP+) (EC 1.1.1.197) is an enzyme that catalyzes the chemical reaction
In enzymology, a 15-hydroxyprostaglandin-I dehydrogenase (NADP+) (EC 1.1.1.231) is an enzyme that catalyzes the chemical reaction
In enzymology, a 15-oxoprostaglandin 13-oxidase (EC 1.3.1.48) is an enzyme that catalyzes the chemical reaction

ALOX15 is, like other lipoxygenases, a seminal enzyme in the metabolism of polyunsaturated fatty acids to a wide range of physiologically and pathologically important products. ▼ Gene Function
In enzymology, a linoleate diol synthase (EC 1.13.11.44) is an enzyme that catalyzes the chemical reaction
In enzymology, a Prostaglandin-A1 Δ-isomerase (EC 5.3.3.9) is an enzyme that catalyzes the chemical reaction

Microsomal prostaglandin E synthase-2 (mPGES-2) or Prostaglandin E synthase 2 is an enzyme that in humans encoded by the PTGES2 gene located on chromosome 9. The protein encoded by this gene is a membrane-associated prostaglandin E synthase, which catalyzes the conversion of prostaglandin H2 to prostaglandin E2. This protein also has been shown to activate the transcription regulated by a gamma-interferon-activated transcription element (GATE). Multiple transcript variants have been found for this gene.
Prostamide/prostaglandin F2alpha synthase is an enzyme with systematic name thioredoxin:(5Z,9alpha,11alpha,13E,15S)-9,11-epidioxy-15-hydroxy-prosta-5,13-dienoate oxidoreductase . This enzyme catalyses the following chemical reaction
9,12-octadecadienoate 8-hydroperoxide 8R-isomerase is an enzyme with systematic name (8R,9Z,12Z)-8-hydroperoxyoctadeca-9,12-dienoate hydroxymutase ( -5,8-dihydroxyoctadeca-9,12-dienoate-forming). This enzyme catalyses the following chemical reaction
9,12-octadecadienoate 8-hydroperoxide 8S-isomerase is an enzyme with systematic name (8R,9Z,12Z)-8-hydroperoxyoctadeca-9,12-dienoate hydroxymutase ( -7,8-dihydroxyoctadeca-9,12-dienoate-forming). This enzyme catalyses the following chemical reaction
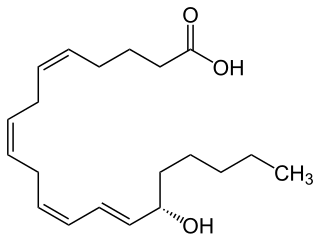
15-Hydroxyeicosatetraenoic acid (also termed 15-HETE, 15(S)-HETE, and 15S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types metabolize arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HpETE). This initial hydroperoxide product is extremely short-lived in cells: if not otherwise metabolized, it is rapidly reduced to 15(S)-HETE. Both of these metabolites, depending on the cell type which forms them, can be further metabolized to 15-oxo-eicosatetraenoic acid (15-oxo-ETE), 5(S),15(S)-dihydroxy-eicosatetraenoic acid (5(S),15(S)-diHETE), 5-oxo-15(S)-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE), a subset of specialized pro-resolving mediators viz., the lipoxins, a class of pro-inflammatory mediators, the eoxins, and other products that have less well-defined activities and functions. Thus, 15(S)-HETE and 15(S)-HpETE, in addition to having intrinsic biological activities, are key precursors to numerous biologically active derivatives.
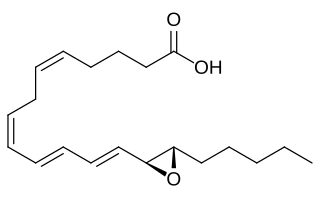
Eoxins are proposed to be a family of proinflammatory eicosanoids. They are produced by human eosinophils, mast cells, the L1236 Reed–Sternberg cell line derived from Hodgkin's lymphoma, and certain other tissues. These cells produce the eoxins by initially metabolizing arachidonic acid, an omega-6 (ω-6) fatty acid, via any enzyme possessing 15-lipoxygenase activity. The product of this initial metabolic step, 15(S)-hydroperoxyeicosatetraenoic acid, is then converted to a series of eoxins by the same enzymes that metabolize the 5-lipoxygenase product of arachidonic acid metabolism, i.e. 5-Hydroperoxy-eicosatetraenoic acid to a series of leukotrienes. That is, the eoxins are 14,15-disubstituted analogs of the 5,6-disubstituted leukotrienes.

Setipiprant (INN; developmental code names ACT-129968, KYTH-105) is an investigational drug developed for the treatment of asthma and scalp hair loss. It was originally developed by Actelion and acts as a selective, orally available antagonist of the prostaglandin D2 receptor 2 (DP2). The drug is being developed as a novel treatment for male pattern baldness by Allergan.
In enzymology, a prostaglandin-F synthase (PGFS; EC 1.1.1.188) is an enzyme that catalyzes the chemical reaction:










