
The inner ear is the innermost part of the vertebrate ear. In vertebrates, the inner ear is mainly responsible for sound detection and balance. In mammals, it consists of the bony labyrinth, a hollow cavity in the temporal bone of the skull with a system of passages comprising two main functional parts:

The cochlea is the part of the inner ear involved in hearing. It is a spiral-shaped cavity in the bony labyrinth, in humans making 2.75 turns around its axis, the modiolus. A core component of the cochlea is the organ of Corti, the sensory organ of hearing, which is distributed along the partition separating the fluid chambers in the coiled tapered tube of the cochlea.
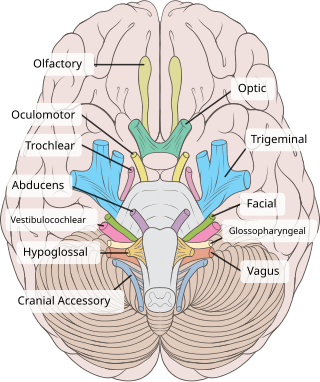
The vestibulocochlear nerve or auditory vestibular nerve, also known as the eighth cranial nerve, cranial nerve VIII, or simply CN VIII, is a cranial nerve that transmits sound and equilibrium (balance) information from the inner ear to the brain. Through olivocochlear fibers, it also transmits motor and modulatory information from the superior olivary complex in the brainstem to the cochlea.

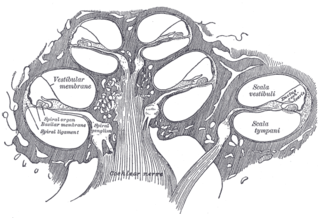
The basilar membrane is a stiff structural element within the cochlea of the inner ear which separates two liquid-filled tubes that run along the coil of the cochlea, the scala media and the scala tympani. The basilar membrane moves up and down in response to incoming sound waves, which are converted to traveling waves on the basilar membrane.

The organ of Corti, or spiral organ, is the receptor organ for hearing and is located in the mammalian cochlea. This highly varied strip of epithelial cells allows for transduction of auditory signals into nerve impulses' action potential. Transduction occurs through vibrations of structures in the inner ear causing displacement of cochlear fluid and movement of hair cells at the organ of Corti to produce electrochemical signals.

The auditory system is the sensory system for the sense of hearing. It includes both the sensory organs and the auditory parts of the sensory system.
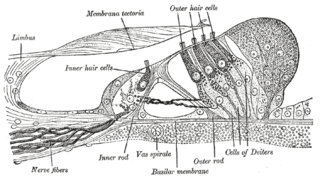
Hair cells are the sensory receptors of both the auditory system and the vestibular system in the ears of all vertebrates, and in the lateral line organ of fishes. Through mechanotransduction, hair cells detect movement in their environment.
In physiology, tonotopy is the spatial arrangement of where sounds of different frequency are processed in the brain. Tones close to each other in terms of frequency are represented in topologically neighbouring regions in the brain. Tonotopic maps are a particular case of topographic organization, similar to retinotopy in the visual system.
Presbycusis, or age-related hearing loss, is the cumulative effect of aging on hearing. It is a progressive and irreversible bilateral symmetrical age-related sensorineural hearing loss resulting from degeneration of the cochlea or associated structures of the inner ear or auditory nerves. The hearing loss is most marked at higher frequencies. Hearing loss that accumulates with age but is caused by factors other than normal aging is not presbycusis, although differentiating the individual effects of distinct causes of hearing loss can be difficult.
In audiology and psychoacoustics the concept of critical bands, introduced by Harvey Fletcher in 1933 and refined in 1940, describes the frequency bandwidth of the "auditory filter" created by the cochlea, the sense organ of hearing within the inner ear. Roughly, the critical band is the band of audio frequencies within which a second tone will interfere with the perception of the first tone by auditory masking.

In the inner ear, stereocilia are the mechanosensing organelles of hair cells, which respond to fluid motion in numerous types of animals for various functions, including hearing and balance. They are about 10–50 micrometers in length and share some similar features of microvilli. The hair cells turn the fluid pressure and other mechanical stimuli into electric stimuli via the many microvilli that make up stereocilia rods. Stereocilia exist in the auditory and vestibular systems.

Volley theory states that groups of neurons of the auditory system respond to a sound by firing action potentials slightly out of phase with one another so that when combined, a greater frequency of sound can be encoded and sent to the brain to be analyzed. The theory was proposed by Ernest Wever and Charles Bray in 1930 as a supplement to the frequency theory of hearing. It was later discovered that this only occurs in response to sounds that are about 500 Hz to 5000 Hz.
The cochlear nerve is one of two parts of the vestibulocochlear nerve, a cranial nerve present in amniotes, the other part being the vestibular nerve. The cochlear nerve carries auditory sensory information from the cochlea of the inner ear directly to the brain. The other portion of the vestibulocochlear nerve is the vestibular nerve, which carries spatial orientation information to the brain from the semicircular canals, also known as semicircular ducts.

The cochlear nucleus (CN) or cochlear nuclear complex comprises two cranial nerve nuclei in the human brainstem, the ventral cochlear nucleus (VCN) and the dorsal cochlear nucleus (DCN). The ventral cochlear nucleus is unlayered whereas the dorsal cochlear nucleus is layered. Auditory nerve fibers, fibers that travel through the auditory nerve carry information from the inner ear, the cochlea, on the same side of the head, to the nerve root in the ventral cochlear nucleus. At the nerve root the fibers branch to innervate the ventral cochlear nucleus and the deep layer of the dorsal cochlear nucleus. All acoustic information thus enters the brain through the cochlear nuclei, where the processing of acoustic information begins. The outputs from the cochlear nuclei are received in higher regions of the auditory brainstem.

The tectoria membrane (TM) is one of two acellular membranes in the cochlea of the inner ear, the other being the basilar membrane (BM). "Tectorial" in anatomy means forming a cover. The TM is located above the spiral limbus and the spiral organ of Corti and extends along the longitudinal length of the cochlea parallel to the BM. Radially the TM is divided into three zones, the limbal, middle and marginal zones. Of these the limbal zone is the thinnest (transversally) and overlies the auditory teeth of Huschke with its inside edge attached to the spiral limbus. The marginal zone is the thickest (transversally) and is divided from the middle zone by Hensen's Stripe. It overlies the sensory inner hair cells and electrically-motile outer hair cells of the organ of Corti and during acoustic stimulation stimulates the inner hair cells through fluid coupling, and the outer hair cells via direct connection to their tallest stereocilia.
The cochlear amplifier is a positive feedback mechanism within the cochlea that provides acute sensitivity in the mammalian auditory system. The main component of the cochlear amplifier is the outer hair cell (OHC) which increases the amplitude and frequency selectivity of sound vibrations using electromechanical feedback.
Electrocochleography is a technique of recording electrical potentials generated in the inner ear and auditory nerve in response to sound stimulation, using an electrode placed in the ear canal or tympanic membrane. The test is performed by an otologist or audiologist with specialized training, and is used for detection of elevated inner ear pressure or for the testing and monitoring of inner ear and auditory nerve function during surgery.
Cochlea is Latin for “snail, shell or screw” and originates from the Greek word κοχλίας kokhlias. The modern definition, the auditory portion of the inner ear, originated in the late 17th century. Within the mammalian cochlea exists the organ of Corti, which contains hair cells that are responsible for translating the vibrations it receives from surrounding fluid-filled ducts into electrical impulses that are sent to the brain to process sound.
Temporal envelope (ENV) and temporal fine structure (TFS) are changes in the amplitude and frequency of sound perceived by humans over time. These temporal changes are responsible for several aspects of auditory perception, including loudness, pitch and timbre perception and spatial hearing.

A. James Hudspeth is the F.M. Kirby Professor at Rockefeller University in New York City, where he is director of the F.M. Kirby Center for Sensory Neuroscience. His laboratory studies the physiological basis of hearing.
















