
Hemoglobin is a protein containing iron that facilitates the transport of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the exception of the fish family Channichthyidae and the tissues of some invertebrate animals. Hemoglobin in the blood carries oxygen from the respiratory organs to the other tissues of the body, where it releases the oxygen to enable aerobic respiration which powers the animal's metabolism. A healthy human has 12 to 20 grams of hemoglobin in every 100 mL of blood. Hemoglobin is a metalloprotein, a chromoprotein, and globulin.

Myelin is a lipid-rich extramembranous structure found on the axons and dendrites of neuron in many bilaterian animals, mainly vertebrates, as well as some arthropods and annelids. A circumferential wrapping of myelin, known as a myelin sheath, increases the conduction speed of electrical impulses passing along the axon by generating saltatory conductions, which are much faster than conduction along an unmyelinated axon, and also reduce signal loss due to extrinsic disturbances.
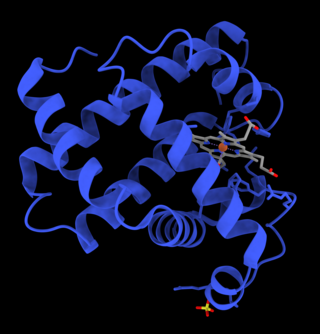
Myoglobin is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobin has a higher affinity for oxygen and does not have cooperative binding with oxygen like hemoglobin does. Myoglobin consists of non-polar amino acids at the core of the globulin, where the heme group is non-covalently bounded with the surrounding polypeptide of myoglobin. In humans, myoglobin is only found in the bloodstream after muscle injury.

A hemeprotein, or heme protein, is a protein that contains a heme prosthetic group. They are a very large class of metalloproteins. The heme group confers functionality, which can include oxygen carrying, oxygen reduction, electron transfer, and other processes. Heme is bound to the protein either covalently or noncovalently or both.

Heme, or haem, is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.

The globins are a superfamily of heme-containing globular proteins, involved in binding and/or transporting oxygen. These proteins all incorporate the globin fold, a series of eight alpha helical segments. Two prominent members include myoglobin and hemoglobin. Both of these proteins reversibly bind oxygen via a heme prosthetic group. They are widely distributed in many organisms.

Hemolymph, or haemolymph, is a fluid, analogous to the blood in vertebrates, that circulates in the interior of the arthropod (invertebrate) body, remaining in direct contact with the animal's tissues. It is composed of a fluid plasma in which hemolymph cells called hemocytes are suspended. In addition to hemocytes, the plasma also contains many chemicals. It is the major tissue type of the open circulatory system characteristic of arthropods. In addition, some non-arthropods such as mollusks possess a hemolymphatic circulatory system.

Glia, also called glial cells(gliocytes) or neuroglia, are non-neuronal cells in the central nervous system (brain and spinal cord) and the peripheral nervous system that do not produce electrical impulses. The neuroglia make up more than one half the volume of neural tissue in our body. They maintain homeostasis, form myelin in the peripheral nervous system, and provide support and protection for neurons. In the central nervous system, glial cells include oligodendrocytes, astrocytes, ependymal cells and microglia, and in the peripheral nervous system they include Schwann cells and satellite cells.
Carboxyhemoglobin is a stable complex of carbon monoxide and hemoglobin (Hb) that forms in red blood cells upon contact with carbon monoxide. Carboxyhemoglobin is often mistaken for the compound formed by the combination of carbon dioxide (carboxyl) and hemoglobin, which is actually carbaminohemoglobin. Carboxyhemoglobin terminology emerged when carbon monoxide was known by its historic name, "carbonic oxide", and evolved through Germanic and British English etymological influences; the preferred IUPAC nomenclature is carbonylhemoglobin.

Astrocytes, also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of endothelial cells that form the blood–brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, regulation of cerebral blood flow, and a role in the repair and scarring process of the brain and spinal cord following infection and traumatic injuries. The proportion of astrocytes in the brain is not well defined; depending on the counting technique used, studies have found that the astrocyte proportion varies by region and ranges from 20% to around 40% of all glia. Another study reports that astrocytes are the most numerous cell type in the brain. Astrocytes are the major source of cholesterol in the central nervous system. Apolipoprotein E transports cholesterol from astrocytes to neurons and other glial cells, regulating cell signaling in the brain. Astrocytes in humans are more than twenty times larger than in rodent brains, and make contact with more than ten times the number of synapses.
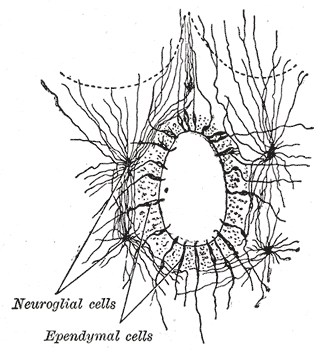
The ependyma is the thin neuroepithelial lining of the ventricular system of the brain and the central canal of the spinal cord. The ependyma is one of the four types of neuroglia in the central nervous system (CNS). It is involved in the production of cerebrospinal fluid (CSF), and is shown to serve as a reservoir for neuroregeneration.

Astrogliosis is an abnormal increase in the number of astrocytes due to the destruction of nearby neurons from central nervous system (CNS) trauma, infection, ischemia, stroke, autoimmune responses or neurodegenerative disease. In healthy neural tissue, astrocytes play critical roles in energy provision, regulation of blood flow, homeostasis of extracellular fluid, homeostasis of ions and transmitters, regulation of synapse function and synaptic remodeling. Astrogliosis changes the molecular expression and morphology of astrocytes, in response to infection for example, in severe cases causing glial scar formation that may inhibit axon regeneration.
A respiratory pigment is a metalloprotein that serves a variety of important functions, its main being O2 transport. Other functions performed include O2 storage, CO2 transport, and transportation of substances other than respiratory gases. There are four major classifications of respiratory pigment: hemoglobin, hemocyanin, erythrocruorin–chlorocruorin, and hemerythrin. The heme-containing globin is the most commonly-occurring respiratory pigment, occurring in at least 9 different phyla of animals.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

The glia limitans, or the glial limiting membrane, is a thin barrier of astrocyte foot processes associated with the parenchymal basal lamina surrounding the brain and spinal cord. It is the outermost layer of neural tissue, and among its responsibilities is the prevention of the over-migration of neurons and neuroglia, the supporting cells of the nervous system, into the meninges. The glia limitans also plays an important role in regulating the movement of small molecules and cells into the brain tissue by working in concert with other components of the central nervous system (CNS) such as the blood–brain barrier (BBB).
Glucose transporter 3, also known as solute carrier family 2, facilitated glucose transporter member 3 (SLC2A3) is a protein that in humans is encoded by the SLC2A3 gene. GLUT3 facilitates the transport of glucose across the plasma membranes of mammalian cells. GLUT3 is most known for its specific expression in neurons and has originally been designated as the neuronal GLUT. GLUT3 has been studied in other cell types with specific glucose requirements, including sperm, preimplantation embryos, circulating white blood cells and carcinoma cell lines.

Cytoglobin is the protein product of CYGB, a human and mammalian gene.

Brain mitochondrial carrier protein 1 is a protein that in humans is encoded by the SLC25A14 gene.
Superficial hemosiderosis of the central nervous system is a disease of the brain resulting from chronic iron deposition in neuronal tissues associated with cerebrospinal fluid. This occurs via the deposition of hemosiderin in neuronal tissue, and is associated with neuronal loss, gliosis, and demyelination of neuronal cells. This disease was first discovered in 1908 by R.C. Hamill after performing an autopsy. Detection of this disease was largely post-mortem until the advent of MRI technology, which made diagnosis far easier. Superficial siderosis is largely considered a rare disease, with less than 270 total reported cases in scientific literature as of 2006, and affects people of a wide range of ages with men being approximately three times more frequently affected than women. The number of reported cases of superficial siderosis has increased with advances in MRI technology, but it remains a rare disease.
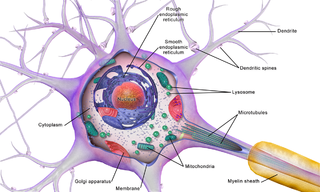
Brain cells make up the functional tissue of the brain. The rest of the brain tissue is structural or connective called the stroma which includes blood vessels. The two main types of cells in the brain are neurons, also known as nerve cells, and glial cells, also known as neuroglia.















