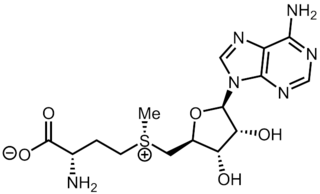
S-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952.

Thiopurine methyltransferase or thiopurine S-methyltransferase (TPMT) is an enzyme that in humans is encoded by the TPMT gene. A pseudogene for this locus is located on chromosome 18q.

Glucose transporter type 4 (GLUT4), also known as solute carrier family 2, facilitated glucose transporter member 4, is a protein encoded, in humans, by the SLC2A4 gene. GLUT4 is the insulin-regulated glucose transporter found primarily in adipose tissues and striated muscle. The first evidence for this distinct glucose transport protein was provided by David James in 1988. The gene that encodes GLUT4 was cloned and mapped in 1989.

Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Rossmann fold for binding S-Adenosyl methionine (SAM). Class II methyltransferases contain a SET domain, which are exemplified by SET domain histone methyltransferases, and class III methyltransferases, which are membrane associated. Methyltransferases can also be grouped as different types utilizing different substrates in methyl transfer reactions. These types include protein methyltransferases, DNA/RNA methyltransferases, natural product methyltransferases, and non-SAM dependent methyltransferases. SAM is the classical methyl donor for methyltransferases, however, examples of other methyl donors are seen in nature. The general mechanism for methyl transfer is a SN2-like nucleophilic attack where the methionine sulfur serves as the leaving group and the methyl group attached to it acts as the electrophile that transfers the methyl group to the enzyme substrate. SAM is converted to S-Adenosyl homocysteine (SAH) during this process. The breaking of the SAM-methyl bond and the formation of the substrate-methyl bond happen nearly simultaneously. These enzymatic reactions are found in many pathways and are implicated in genetic diseases, cancer, and metabolic diseases. Another type of methyl transfer is the radical S-Adenosyl methionine (SAM) which is the methylation of unactivated carbon atoms in primary metabolites, proteins, lipids, and RNA.

N-Acetylserotonin O-methyltransferase, also known as ASMT, is an enzyme which catalyzes the final reaction in melatonin biosynthesis: converting Normelatonin to melatonin. This reaction is embedded in the more general tryptophan metabolism pathway. The enzyme also catalyzes a second reaction in tryptophan metabolism: the conversion of 5-hydroxy-indoleacetate to 5-methoxy-indoleacetate. The other enzyme which catalyzes this reaction is n-acetylserotonin-o-methyltransferase-like-protein.

Hypermethioninemia is an excess of the amino acid methionine, in the blood. This condition can occur when methionine is not broken down properly in the body.

Histamine N-methyltransferase (HNMT) is a protein encoded by the HNMT gene in humans. It belongs to the methyltransferases superfamily of enzymes and plays a role in the inactivation of histamine, a biomolecule that is involved in various physiological processes. Methyltransferases are present in every life form including archaeans, with 230 families of methyltransferases found across species.
Amine N-methyltransferase, also called indolethylamine N-methyltransferase, and thioether S-methyltransferase, is an enzyme that is ubiquitously present in non-neural tissues and catalyzes the N-methylation of tryptamine and structurally related compounds. More recently, it was discovered that this enzyme can also catalyze the methylation of thioether and selenoether compounds, although the physiological significance of this biotransformation is not yet known.

In enzymology, a caffeate O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a carnosine N-methyltransferase is an enzyme that catalyzes the chemical reaction

Guanidinoacetate N-methyltransferase is an enzyme that catalyzes the chemical reaction and is encoded by gene GAMT located on chromosome 19p13.3.
In enzymology, an indolepyruvate C-methyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a nicotinate N-methyltransferase is an enzyme that catalyzes the chemical reaction

Phosphatidylethanolamine N-methyltransferase is a transferase enzyme which converts phosphatidylethanolamine (PE) to phosphatidylcholine (PC) in the liver. In humans it is encoded by the PEMT gene within the Smith–Magenis syndrome region on chromosome 17.
In enzymology, a phosphatidyl-N-methylethanolamine N-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a precorrin-4 C11-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a thiol S-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a tRNA (guanine-N2-)-methyltransferase (EC 2.1.1.32) is an enzyme that catalyzes the chemical reaction
Nicotinamide N-methyltransferase (NNMT) is an enzyme that in humans is encoded by the NNMT gene. NNMT catalyzes the methylation of nicotinamide and similar compounds using the methyl donor S-adenosyl methionine (SAM-e) to produce S-adenosyl-L-homocysteine (SAH) and 1-methylnicotinamide.

1-Methylnicotinamide (trigonellamide) is a prototypic organic cation. 1-Methylnicotinamide is the methylated amide of Nicotinamide (niacinamide, vitamin B3).














