
Paecilomyces is a genus of fungi. A number of species in this genus are plant pathogens.
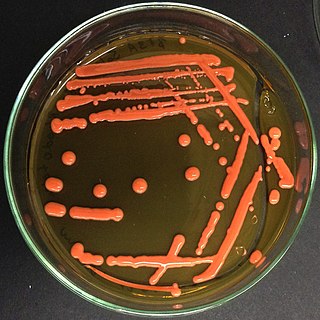
Rhodotorula is a genus of fungi in the class Microbotryomycetes. Most species are known in their yeast states which produce orange to red colonies when grown on Sabouraud's dextrose agar (SDA). The colour is the result of pigments that the yeast creates to block out certain wavelengths of light (620–750 nm) that would otherwise be damaging to the cell. Hyphal states, formerly placed in the genus Rhodosporidium, give rise to teliospores from which laterally septate basidia emerge, producing sessile basidiospores. Species occur worldwide and can be isolated from air, water, soil, and other substrates.
Hyphomycetes are a form classification of fungi, part of what has often been referred to as fungi imperfecti, Deuteromycota, or anamorphic fungi. Hyphomycetes lack closed fruit bodies, and are often referred to as moulds. Most hyphomycetes are now assigned to the Ascomycota, on the basis of genetic connections made by life-cycle studies or by phylogenetic analysis of DNA sequences; many remain unassigned phylogenetically.

Acremonium is a genus of fungi in the family Hypocreaceae. It used to be known as Cephalosporium.

Acrophialophora fusispora is a poorly studied ascomycete fungus found in soil, air and various plants. A. fusispora is morphologically similar to the genera Paecilomyces and Masonia, but differ in the presence of pigmented conidiophores, verticillate phialides, and frequent sympodial proliferation. Moreover, A. fusispora is distinguished by its pigmented spindle-shaped conidia, covered with spiral bands. The fungus is naturally found in soils of tropical to temperate regions. The fungus has been identified as a plant and animal pathogen, and has recently been recognized as an emerging opportunistic human pathogen. A. fusispora infection in human is rare and has few documented clinical cases, but due to the rarity of the fungus and potential misidentification, the infections may be underdiagnosed. Clinical cases of A. fusispora include cases of keratitis, pulmonary colonization and infection, and cerebral infections. The fungus also has two documented cases of infection in dogs.

Purpureocillium lilacinum is a species of filamentous fungus in the family Ophiocordycipitaceae. It has been isolated from a wide range of habitats, including cultivated and uncultivated soils, forests, grassland, deserts, estuarine sediments and sewage sludge, and insects. It has also been found in nematode eggs, and occasionally from females of root-knot and cyst nematodes. In addition, it has frequently been detected in the rhizosphere of many crops. The species can grow at a wide range of temperatures – from 8 to 38 °C for a few isolates, with optimal growth in the range 26 to 30 °C. It also has a wide pH tolerance and can grow on a variety of substrates. P. lilacinum has shown promising results for use as a biocontrol agent to control the growth of destructive root-knot nematodes.
Coniochaeta hoffmannii, also known as Lecythophora hoffmannii, is an ascomycete fungus that grows commonly in soil. It has also been categorized as a soft-rot fungus capable of bringing the surface layer of timber into a state of decay, even when safeguarded with preservatives. Additionally, it has pathogenic properties, although it causes serious infection only in rare cases. A plant pathogen lacking a known sexual state, C. hoffmannii has been classified as a "dematiaceous fungus" despite its contradictory lack of pigmentation; both in vivo and in vitro, there is no correlation between its appearance and its classification.
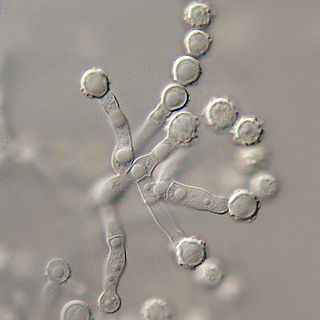
Microascus brevicaulis is a microfungus in the Ascomycota. It is the teleomorph form of Scopulariopsis brevicaulis.Microascus brevicaulis occurs world-wide as a saprotroph in soil, a common agent of biodeterioration, an irregular plant pathogen, and an occasional agent of human nail infection.
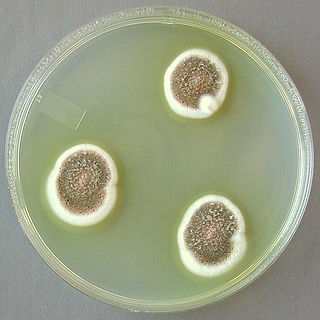
Aspergillus ustus is a microfungus and member of the division Ascomycota. It is commonly found in indoor environments and soil. Isolated cases of human infection resulting from A. ustus have been described; however the majority of these are nail infections.
Phialemonium curvatum is a pathogenic fungus in the phylum Ascomycota. The genus was created to accommodate taxa intermediate to Acremonium and Phialophora. This genus is characterized by its abundance of adelophialides and few discrete phialides with no signs of collarettes. Specifically, P. curvatum is characterized by its grayish white colonies and its allantoid conidia. Phialemonium curvatum is typically found in a variety of environments including air, soil, industrial water and sewage. Furthermore, P. curvatum affects mainly immunocompromised and is rarely seen in immunocompetent people. The species has been known to cause peritonitis, endocarditis, endovascular infections, osteomyelitis as well as cutaneous infections of wounds and burns.

Sporobolomyces salmonicolor is a species of fungus in the subdivision Pucciniomycotina. It occurs in both a yeast state and a hyphal state, the latter formerly known as Sporidiobolus salmonicolor. It is generally considered a Biosafety Risk Group 1 fungus; however isolates of S. salmonicolor have been recovered from cerebrospinal fluid, infected skin, a nasal polyp, lymphadenitis and a case of endophthalmitis. It has also been reported in AIDS-related infections. The fungus exists predominantly in the anamorphic (asexual) state as a unicellular, haploid yeast yet this species can sometimes produce a teleomorphic (sexual) state when conjugation of compatible yeast cells occurs. The asexual form consists of a characteristic, pink, ballistosporic yeast. Ballistoconidia are borne from slender extensions of the cell known as sterigmata and are forcibly ejected into the air upon maturity. Levels of airborne yeast cells peak during the night and are abundant in areas of decaying leaves and grains. Three varieties of Sporobolomyces salmonicolor have been described; S. salmonicolor var. albus, S. salmonicolor var. fischerii, and S. salmonicolor var. salmoneus.

Rhodotorula glutinis is the type species of the genus Rhodotorula, a basidiomycetous genus of pink yeasts which contains 370 species. Heterogeneity of the genus has made its classification difficult with five varieties having been recognized; however, as of 2011, all are considered to represent a single taxon. The fungus is a common colonist of animals, foods and environmental materials. It can cause opportunistic infections, notably blood infection in the setting of significant underlying disease. It has been used industrially in the production of carotenoid pigments and as a biocontrol agent for post-harvest spoilage diseases of fruits.
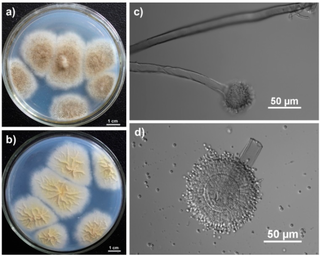
Aspergillus tubingensis is a darkly pigmented species of fungus in the genus Aspergillus section Nigri. It is often confused with Aspergillus niger due to their similar morphology and habitat. A. tubingensis is often involved in food spoilage of fruits and wheat, and industrial fermentation. This species is a rare agent of opportunistic infection.
Paecilomyces marquandii is a soil-borne filamentous fungus distributed throughout temperate to tropical latitudes worldwide including forest, grassland, sewage sludge and strongly metal polluted area characterized by high tolerance in heavy metals. Simultaneous toxic action of zinc and alachlor result an increase in uptake of metal in this fungus but disrupts the cell membrane. Paecilomyces marquandii is known to parasitize the mushroom, Cuphophyllus virgineus, in the family, Hygrophoraceae. Paecilomyces marquandii is categorised as a biosafety risk group 1 in Canada and is not thought to be a significant pathogen of humans or animals.
Penicillium commune is an indoor fungus belonging to the genus Penicillium. It is known as one of the most common fungi spoilage moulds on cheese. It also grows on and spoils other foods such as meat products and fat-containing products like nuts and margarine. Cyclopiazonic acid and regulovasine A and B are the most important mycotoxins produced by P. commune. The fungus is the only known species to be able to produce both penitrem A and roquefortine. Although this species does not produce penicillin, it has shown to have anti-pathogenic activity. There are no known plant, animal or human diseases caused by P. commune.

Penicillium spinulosum is a non-branched, fast-growing fungus with a swelling at the terminal of the stipe (vesiculate) in the genus Penicillium. P. spinulosum is able to grow and reproduce in environment with low temperature and low water availability, and is known to be acidotolerant. P. spinulosum is ubiquitously distributed, and can often be isolated from soil. Each individual strain of P. spinulosum differs from others in their colony morphology, including colony texture, amount of sporulation and roughness of conidia and conidiophores.
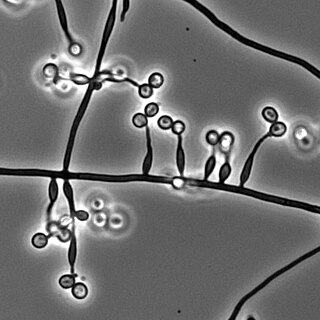
Metarhizium granulomatis is a fungus in the family Clavicipitaceae associated with systemic mycosis in veiled chameleons. The genus Metarhizium is known to infect arthropods, and collectively are referred to green-spored asexual pathogenic fungi. This species grows near the roots of plants and has been reported as an agent of disease in captive veiled chameleons. The etymology of the species epithet, "granulomatis" refers to the ability of the fungus to cause granulomatous disease in susceptible reptiles.
Sarocladium kiliense is a saprobic fungus that is occasionally encountered as a opportunistic pathogen of humans, particularly immunocompromised and individuals. The fungus is frequently found in soil and has been linked with skin and systemic infections. This species is also known to cause disease in the green alga, Cladophora glomerata as well as various fruit and vegetable crops grown in warmer climates.
Aspergillus papuensis is a species of fungus in the genus Aspergillus. It is from the Fumigati section. Several fungi from this section produce heat-resistant ascospores, and the isolates from this section are frequently obtained from locations where natural fires have previously occurred. The species was first described in 2014.
Apinisia keratinophila, formerly Myriodontium keratinophilum, is a fungus widespread in nature, most abundantly found in keratin-rich environments such as feathers, nails and hair. Despite its ability to colonize keratinous surfaces of human body, the species has been known to be non-pathogenic in man and is phylogentically distant to other human pathogenic species, such as anthropophilic dermatophytes. However, its occasional isolation from clinical specimens along with its keratinolytic properties suggest the possibility it may contribute to disease.












