
Lipid-anchored proteins are proteins located on the surface of the cell membrane that are covalently attached to lipids embedded within the cell membrane. These proteins insert and assume a place in the bilayer structure of the membrane alongside the similar fatty acid tails. The lipid-anchored protein can be located on either side of the cell membrane. Thus, the lipid serves to anchor the protein to the cell membrane. They are a type of proteolipids.
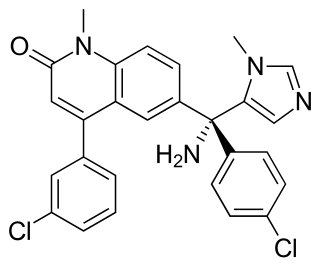
The farnesyltransferase inhibitors (FTIs) are a class of experimental cancer drugs that target protein farnesyltransferase with the downstream effect of preventing the proper functioning of the Ras (protein), which is commonly abnormally active in cancer.

Ribonucleotide reductase (RNR), also known as ribonucleoside diphosphate reductase (rNDP), is an enzyme that catalyzes the formation of deoxyribonucleotides from ribonucleotides. It catalyzes this formation by removing the 2'-hydroxyl group of the ribose ring of nucleoside diphosphates. This reduction produces deoxyribonucleotides. Deoxyribonucleotides in turn are used in the synthesis of DNA. The reaction catalyzed by RNR is strictly conserved in all living organisms. Furthermore, RNR plays a critical role in regulating the total rate of DNA synthesis so that DNA to cell mass is maintained at a constant ratio during cell division and DNA repair. A somewhat unusual feature of the RNR enzyme is that it catalyzes a reaction that proceeds via a free radical mechanism of action. The substrates for RNR are ADP, GDP, CDP and UDP. dTDP is synthesized by another enzyme from dTMP.

Prenylation is the addition of hydrophobic molecules to a protein or a biomolecule. It is usually assumed that prenyl groups (3-methylbut-2-en-1-yl) facilitate attachment to cell membranes, similar to lipid anchors like the GPI anchor, though direct evidence of this has not been observed. Prenyl groups have been shown to be important for protein–protein binding through specialized prenyl-binding domains.
Farnesyltransferase is one of the three enzymes in the prenyltransferase group. Farnesyltransferase (FTase) adds a 15-carbon isoprenoid called a farnesyl group to proteins bearing a CaaX motif: a four-amino acid sequence at the carboxyl terminus of a protein. Farnesyltransferase's targets include members of the Ras superfamily of small GTP-binding proteins critical to cell cycle progression. For this reason, several FTase inhibitors are undergoing testing as anti-cancer agents. FTase inhibitors have shown efficacy as anti-parasitic agents, as well. FTase is also believed to play an important role in development of progeria and various forms of cancers.
Geranylgeranyltransferase type 1 or simply geranylgeranyltransferase is one of the three enzymes in the prenyltransferase group. In specific terms, Geranylgeranyltransferase adds a 20-carbon isoprenoid called a geranylgeranyl group to proteins bearing a CaaX motif: a four-amino acid sequence at the carboxyl terminal of a protein. Geranylgeranyltransferase inhibitors are being investigated as anti-cancer agents.

Rab geranylgeranyltransferase also known as (protein) geranylgeranyltransferase II is one of the three prenyltransferases. It transfers (usually) two geranylgeranyl groups to the cystein(s) at the C-terminus of Rab proteins.
In molecular biology, the Guanosine dissociation inhibitors (GDIs) constitute a family of small GTPases that serve a regulatory role in vesicular membrane traffic. GDIs bind to the GDP-bound form of Rho and Rab small GTPases and not only prevent exchange, but also prevent the small GTPase from localizing at the membrane, which is their place of action. This inhibition can be removed by the action of a GDI displacement factor. GDIs also inhibit cdc42 by binding to its tail and preventing its insertion into membranes; hence it cannot trigger WASPs and cannot lead to nucleation of F-actin.
Geranylgeranylation is a form of prenylation, which is a post-translational modification of proteins that involves the attachment of one or two 20-carbon lipophilic geranylgeranyl isoprene units from geranylgeranyl diphosphate to one or two cysteine residue(s) at the C-terminus of specific proteins. Prenylation is thought to function, at least in part, as a membrane anchor for proteins.

Ras homolog gene family, member B, also known as RHOB, is a protein which in humans is encoded by the RHOB gene.
In enzymology, a di-trans,poly-cis-decaprenylcistransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a farnesyltranstransferase is an enzyme that catalyzes the chemical reaction.
In enzymology, a geranylgeranylglycerol-phosphate geranylgeranyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a geranyltranstransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphoglycerol geranylgeranyltransferase is an enzyme that catalyzes the chemical reaction

Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha is an enzyme that in humans is encoded by the FNTA gene.

Protein farnesyltransferase subunit beta is an enzyme that in humans is encoded by the FNTB gene.
Phytoene synthase is a transferase enzyme involved in the biosynthesis of carotenoids. It catalyzes the conversion of geranylgeranyl pyrophosphate to phytoene. This enzyme catalyses the following chemical reaction
Geranylgeranyl-diphosphate:protein-cysteine geranyltransferase may refer to:

Protein geranylgeranyltransferase type I subunit beta is a protein that in humans is encoded by the PGGT1B gene.










