
Dicofol is an insecticide, an organochlorine that is chemically related to DDT. Dicofol is a miticide that is very effective against spider mite. Its production and use is banned internationally under the Stockholm Convention.

Chlorfenvinphos is an organophosphorus compound that was widely used as an insecticide and an acaricide. The molecule itself can be described as an enol ester derived from dichloroacetophenone and diethylphosphonic acid. Chlorfenvinphos has been included in many products since its first use in 1963. However, because of its toxic effect as a cholinesterase inhibitor it has been banned in several countries, including the United States and the European Union. Its use in the United States was discontinued in 1991.

Ethion (C9H22O4P2S4) is an organophosphate insecticide. It is known to affect the neural enzyme acetylcholinesterase and disrupt its function.
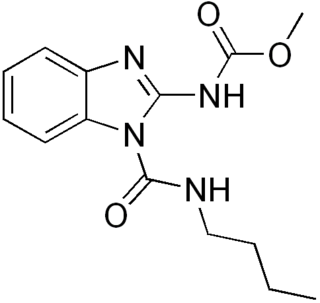
Benomyl is a fungicide introduced in 1968 by DuPont. It is a systemic benzimidazole fungicide that is selectively toxic to microorganisms and invertebrates, but relatively nontoxic toward mammals.

Heptachlor is an organochlorine compound that was used as an insecticide. Usually sold as a white or tan powder, heptachlor is one of the cyclodiene insecticides. In 1962, Rachel Carson's Silent Spring questioned the safety of heptachlor and other chlorinated insecticides. Due to its highly stable structure, heptachlor can persist in the environment for decades. In the United States, the Environmental Protection Agency has limited the sale of heptachlor products to the specific application of fire ant control in underground transformers. The amount that can be present in different foods is regulated.

Phosmet is a phthalimide-derived, non-systemic, organophosphate insecticide used on plants and animals. It is mainly used on apple trees for control of codling moth, though it is also used on a wide range of fruit crops, ornamentals, and vines for the control of aphids, suckers, mites, and fruit flies.

Dimethoate is a widely used organophosphate insecticide and acaricide. It was patented and introduced in the 1950s by American Cyanamid. Like other organophosphates, dimethoate is an acetylcholinesterase inhibitor which disables cholinesterase, an enzyme essential for central nervous system function. It acts both by contact and through ingestion. It is readily absorbed and distributed throughout plant tissues, and is degraded relatively rapidly. One of the breakdown products of dimethoate is omethoate, a potent cholinesterase inhibitor, is ten times more toxic than its parent compound.

Methiocarb is a carbamate pesticide which is used as an insecticide, bird repellent, acaricide and molluscicide since the 1960s. Methiocarb has contact and stomach action on mites and neurotoxic effects on molluscs. Seeds treated with methiocarb also affect birds. Other names for methiocarb are mesurol and mercaptodimethur.

Acetamiprid is an organic compound with the chemical formula C10H11ClN4. It is an odorless neonicotinoid insecticide produced under the trade names Assail, and Chipco by Aventis CropSciences. It is systemic and intended to control sucking insects (Thysanoptera, Hemiptera, mainly aphids) on crops such as leafy vegetables, citrus fruits, pome fruits, grapes, cotton, cole crops, and ornamental plants. It is also a key pesticide in commercial cherry farming due to its effectiveness against the larvae of the cherry fruit fly.
Dimethyl tetrachloroterephthalate (DCPA, with the main trade name Dacthal) is an organic compound with the formula C6Cl4(CO2CH3)2. It is the dimethyl ester of tetrachloroterephthalic acid, used as a preemergent herbicide with the ISO common name chlorthal-dimethyl. It kills annual grasses and many common weeds without killing sensitive plants such as turf grasses, flowers, fruits, vegetables, and cotton.

Carbophenothion also known as Stauffer R 1303 as for the manufacturer, Stauffer Chemical, is an organophosphorus chemical compound. It was used as a pesticide for citrus fruits under the name of Trithion. Carbophenothion was used as an insecticide and acaricide. Although not used anymore it is still a restricted use pesticide in the United States. The chemical is identified in the US as an extremely hazardous substance according to the Emergency Planning and Community Right-to-Know Act.

Ethoprophos (or ethoprop) is an organophosphate ester with the formula C8H19O2PS2. It is a clear yellow to colourless liquid that has a characteristic mercaptan-like odour. It is used as an insecticide and nematicide and it is an acetylcholinesterase inhibitor.
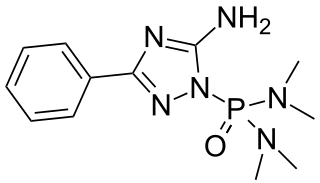
Triamiphos (chemical formula: C12H19N6OP) is an organophosphate used as a pesticide and fungicide. It is used to control powdery mildews on apples and ornamentals. It was discontinued by the US manufacturer in 1998.

Triazofos is a chemical compound used in acaricides, insecticides, and nematicides.

Imazaquin is an imidazolinone herbicide, so named because it contains an imidazolinone core. This organic compound is used to control a broad spectrum of weed species. It is a colorless or white solid, although commercial samples can appear brown or tan.

Cyproconazole is an agricultural fungicide of the class of azoles, used on cereal crops, coffee, sugar beet, fruit trees and grapes, and peanuts, on sod farms and golf course turf and on wood as a preservative. It has been used against powdery mildew, rust on cereals and apple scab, and applied by air or on the ground or by chemigation.

Tebufenpyrad is an insecticide and acaricide widely used in greenhouses. It is a white solid chemical with a slight aromatic smell. It is soluble in water and also in organic solvents.

Cyanazine is a herbicide that belongs to the group of triazines. Cyanazine inhibits photosynthesis and is therefore used as a herbicide.
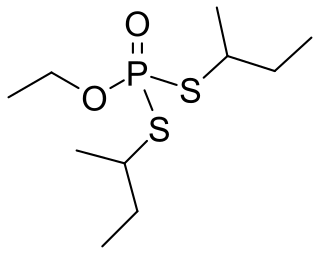
Cadusafos is a chemical insecticide and nematicide often used against parasitic nematode populations. The compound acts as a acetylcholinesterase inhibitor. It belongs the chemical class of synthetic organic thiophosphates and it is a volatile and persistent clear liquid. It is used on food crops such as tomatoes, bananas and chickpeas. It is currently not approved by the European Commission for use in the EU. Exposure can occur through inhalation, ingestion or contact with the skin. The compound is highly toxic to nematodes, earthworms and birds but poses no carcinogenic risk to humans.




















