Related Research Articles
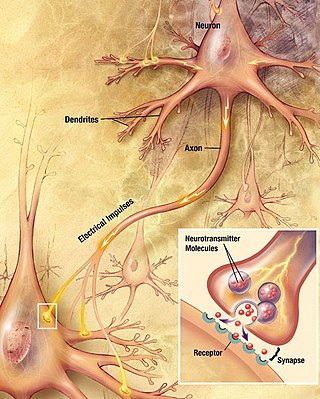
Chemical synapses are biological junctions through which neurons' signals can be sent to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous system. They are crucial to the biological computations that underlie perception and thought. They allow the nervous system to connect to and control other systems of the body.

In neuroscience, long-term potentiation (LTP) is a persistent strengthening of synapses based on recent patterns of activity. These are patterns of synaptic activity that produce a long-lasting increase in signal transmission between two neurons. The opposite of LTP is long-term depression, which produces a long-lasting decrease in synaptic strength.
In neuroscience, synaptic plasticity is the ability of synapses to strengthen or weaken over time, in response to increases or decreases in their activity. Since memories are postulated to be represented by vastly interconnected neural circuits in the brain, synaptic plasticity is one of the important neurochemical foundations of learning and memory.

In neuroscience, an excitatory postsynaptic potential (EPSP) is a postsynaptic potential that makes the postsynaptic neuron more likely to fire an action potential. This temporary depolarization of postsynaptic membrane potential, caused by the flow of positively charged ions into the postsynaptic cell, is a result of opening ligand-gated ion channels. These are the opposite of inhibitory postsynaptic potentials (IPSPs), which usually result from the flow of negative ions into the cell or positive ions out of the cell. EPSPs can also result from a decrease in outgoing positive charges, while IPSPs are sometimes caused by an increase in positive charge outflow. The flow of ions that causes an EPSP is an excitatory postsynaptic current (EPSC).
In neurophysiology, long-term depression (LTD) is an activity-dependent reduction in the efficacy of neuronal synapses lasting hours or longer following a long patterned stimulus. LTD occurs in many areas of the CNS with varying mechanisms depending upon brain region and developmental progress.
In neuroscience, a silent synapse is an excitatory glutamatergic synapse whose postsynaptic membrane contains NMDA-type glutamate receptors but no AMPA-type glutamate receptors. These synapses are named "silent" because normal AMPA receptor-mediated signaling is not present, rendering the synapse inactive under typical conditions. Silent synapses are typically considered to be immature glutamatergic synapses. As the brain matures, the relative number of silent synapses decreases. However, recent research on hippocampal silent synapses shows that while they may indeed be a developmental landmark in the formation of a synapse, that synapses can be "silenced" by activity, even once they have acquired AMPA receptors. Thus, silence may be a state that synapses can visit many times during their lifetimes.
Spike-timing-dependent plasticity (STDP) is a biological process that adjusts the strength of connections between neurons in the brain. The process adjusts the connection strengths based on the relative timing of a particular neuron's output and input action potentials. The STDP process partially explains the activity-dependent development of nervous systems, especially with regard to long-term potentiation and long-term depression.
An apical dendrite is a dendrite that emerges from the apex of a pyramidal cell. Apical dendrites are one of two primary categories of dendrites, and they distinguish the pyramidal cells from spiny stellate cells in the cortices. Pyramidal cells are found in the prefrontal cortex, the hippocampus, the entorhinal cortex, the olfactory cortex, and other areas. Dendrite arbors formed by apical dendrites are the means by which synaptic inputs into a cell are integrated. The apical dendrites in these regions contribute significantly to memory, learning, and sensory associations by modulating the excitatory and inhibitory signals received by the pyramidal cells.
Depolarization-induced suppression of inhibition is the classical and original electrophysiological example of endocannabinoid function in the central nervous system. Prior to the demonstration that depolarization-induced suppression of inhibition was dependent on the cannabinoid CB1 receptor function, there was no way of producing an in vitro endocannabinoid mediated effect.
Metaplasticity is a term originally coined by W.C. Abraham and M.F. Bear to refer to the plasticity of synaptic plasticity. Until that time synaptic plasticity had referred to the plastic nature of individual synapses. However this new form referred to the plasticity of the plasticity itself, thus the term meta-plasticity. The idea is that the synapse's previous history of activity determines its current plasticity. This may play a role in some of the underlying mechanisms thought to be important in memory and learning such as long-term potentiation (LTP), long-term depression (LTD) and so forth. These mechanisms depend on current synaptic "state", as set by ongoing extrinsic influences such as the level of synaptic inhibition, the activity of modulatory afferents such as catecholamines, and the pool of hormones affecting the synapses under study. Recently, it has become clear that the prior history of synaptic activity is an additional variable that influences the synaptic state, and thereby the degree, of LTP or LTD produced by a given experimental protocol. In a sense, then, synaptic plasticity is governed by an activity-dependent plasticity of the synaptic state; such plasticity of synaptic plasticity has been termed metaplasticity. There is little known about metaplasticity, and there is much research currently underway on the subject, despite its difficulty of study, because of its theoretical importance in brain and cognitive science. Most research of this type is done via cultured hippocampus cells or hippocampal slices.

In the nervous system, a synapse is a structure that permits a neuron to pass an electrical or chemical signal to another neuron or to the target effector cell.

In the hippocampus, the mossy fiber pathway consists of unmyelinated axons projecting from granule cells in the dentate gyrus that terminate on modulatory hilar mossy cells and in Cornu Ammonis area 3 (CA3), a region involved in encoding short-term memory. These axons were first described as mossy fibers by Santiago Ramón y Cajal as they displayed varicosities along their lengths that gave them a mossy appearance. The axons that make up the pathway emerge from the basal portions of the granule cells and pass through the hilus of the dentate gyrus before entering the stratum lucidum of CA3. Granule cell synapses tend to be glutamatergic, though immunohistological data has indicated that some synapses contain neuropeptidergic elements including opiate peptides such as dynorphin and enkephalin. There is also evidence for co-localization of both GABAergic and glutamatergic neurotransmitters within mossy fiber terminals. GABAergic and glutamatergic co-localization in mossy fiber boutons has been observed primarily in the developing hippocampus, but in adulthood, evidence suggests that mossy fiber synapses may alternate which neurotransmitter is released through activity-dependent regulation.

Synaptic potential refers to the potential difference across the postsynaptic membrane that results from the action of neurotransmitters at a neuronal synapse. In other words, it is the “incoming” signal that a neuron receives. There are two forms of synaptic potential: excitatory and inhibitory. The type of potential produced depends on both the postsynaptic receptor, more specifically the changes in conductance of ion channels in the post synaptic membrane, and the nature of the released neurotransmitter. Excitatory post-synaptic potentials (EPSPs) depolarize the membrane and move the potential closer to the threshold for an action potential to be generated. Inhibitory postsynaptic potentials (IPSPs) hyperpolarize the membrane and move the potential farther away from the threshold, decreasing the likelihood of an action potential occurring. The Excitatory Post Synaptic potential is most likely going to be carried out by the neurotransmitters glutamate and acetylcholine, while the Inhibitory post synaptic potential will most likely be carried out by the neurotransmitters gamma-aminobutyric acid (GABA) and glycine. In order to depolarize a neuron enough to cause an action potential, there must be enough EPSPs to both depolarize the postsynaptic membrane from its resting membrane potential to its threshold and counterbalance the concurrent IPSPs that hyperpolarize the membrane. As an example, consider a neuron with a resting membrane potential of -70 mV (millivolts) and a threshold of -50 mV. It will need to be raised 20 mV in order to pass the threshold and fire an action potential. The neuron will account for all the many incoming excitatory and inhibitory signals via summative neural integration, and if the result is an increase of 20 mV or more, an action potential will occur.
Coincidence detection is a neuronal process in which a neural circuit encodes information by detecting the occurrence of temporally close but spatially distributed input signals. Coincidence detectors influence neuronal information processing by reducing temporal jitter and spontaneous activity, allowing the creation of variable associations between separate neural events in memory. The study of coincidence detectors has been crucial in neuroscience with regards to understanding the formation of computational maps in the brain.

In neurophysiology, a dendritic spike refers to an action potential generated in the dendrite of a neuron. Dendrites are branched extensions of a neuron. They receive electrical signals emitted from projecting neurons and transfer these signals to the cell body, or soma. Dendritic signaling has traditionally been viewed as a passive mode of electrical signaling. Unlike its axon counterpart which can generate signals through action potentials, dendrites were believed to only have the ability to propagate electrical signals by physical means: changes in conductance, length, cross sectional area, etc. However, the existence of dendritic spikes was proposed and demonstrated by W. Alden Spencer, Eric Kandel, Rodolfo Llinás and coworkers in the 1960s and a large body of evidence now makes it clear that dendrites are active neuronal structures. Dendrites contain voltage-gated ion channels giving them the ability to generate action potentials. Dendritic spikes have been recorded in numerous types of neurons in the brain and are thought to have great implications in neuronal communication, memory, and learning. They are one of the major factors in long-term potentiation.

Nonsynaptic plasticity is a form of neuroplasticity that involves modification of ion channel function in the axon, dendrites, and cell body that results in specific changes in the integration of excitatory postsynaptic potentials and inhibitory postsynaptic potentials. Nonsynaptic plasticity is a modification of the intrinsic excitability of the neuron. It interacts with synaptic plasticity, but it is considered a separate entity from synaptic plasticity. Intrinsic modification of the electrical properties of neurons plays a role in many aspects of plasticity from homeostatic plasticity to learning and memory itself. Nonsynaptic plasticity affects synaptic integration, subthreshold propagation, spike generation, and other fundamental mechanisms of neurons at the cellular level. These individual neuronal alterations can result in changes in higher brain function, especially learning and memory. However, as an emerging field in neuroscience, much of the knowledge about nonsynaptic plasticity is uncertain and still requires further investigation to better define its role in brain function and behavior.
In neuroscience, synaptic scaling is a form of homeostatic plasticity, in which the brain responds to chronically elevated activity in a neural circuit with negative feedback, allowing individual neurons to reduce their overall action potential firing rate. Where Hebbian plasticity mechanisms modify neural synaptic connections selectively, synaptic scaling normalizes all neural synaptic connections by decreasing the strength of each synapse by the same factor, so that the relative synaptic weighting of each synapse is preserved.

Rapastinel (INN) is a novel antidepressant that was under development by Allergan as an adjunctive therapy for the treatment of treatment-resistant depression. It is a centrally active, intravenously administered amidated tetrapeptide that acts as a novel and selective modulator of the NMDA receptor. The drug is a rapid-acting and long-lasting antidepressant as well as robust cognitive enhancer by virtue of its ability to enhance NMDA receptor-mediated signal transduction and synaptic plasticity.
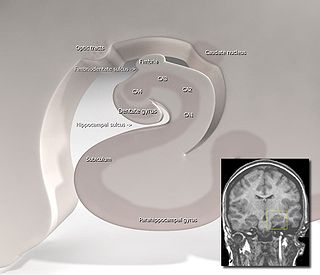
The hippocampus proper refers to the actual structure of the hippocampus which is made up of three regions or subfields. The subfields CA1, CA2, and CA3 use the initials of cornu Ammonis, an earlier name of the hippocampus.
Early long-term potentiation (E-LTP) is the first phase of long-term potentiation (LTP), a well-studied form of synaptic plasticity, and consists of an increase in synaptic strength. LTP could be produced by repetitive stimulation of the presynaptic terminals, and it is believed to play a role in memory function in the hippocampus, amygdala and other cortical brain structures in mammals.
References
- ↑ Vago, David R.; Kesner, Raymond P. (June 2008). "Disruption of the direct perforant path input to the CA1 subregion of the dorsal hippocampus interferes with spatial working memory and novelty detection". Behavioural Brain Research. 189 (2): 273–283. doi:10.1016/j.bbr.2008.01.002. PMC 2421012 . PMID 18313770.
- 1 2 3 Schiess, Adrian R. B.; Scullin, Chessa; Donald Partridge, L. (April 2010). "Maturation of Schaffer collateral synapses generates a phenotype of unreliable basal evoked release and very reliable facilitated release". European Journal of Neuroscience. 31 (8): 1377–1387. doi:10.1111/j.1460-9568.2010.07180.x. PMC 3575738 . PMID 20384768.
- ↑ "arborization". The Free Dictionary.
- ↑ Smith, M. A; Ellis-Davies, G. C R; Magee, J. C (21 February 2003). "Mechanism of the distance-dependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons". The Journal of Physiology. 548 (1): 245–258. doi:10.1113/jphysiol.2002.036376. PMC 2342790 . PMID 12598591.
- ↑ Lebeau, Geneviève; DesGroseillers, Luc; Sossin, Wayne; Lacaille, Jean-Claude (2011). "mRNA binding protein staufen 1-dependent regulation of pyramidal cell spine morphology via NMDA receptor-mediated synaptic plasticity". Molecular Brain. 4 (1): 22. doi: 10.1186/1756-6606-4-22 . PMC 3118231 . PMID 21635779.
- ↑ Arrigoni, Elda; Greene, Robert W (May 2004). "Schaffer collateral and perforant path inputs activate different subtypes of NMDA receptors on the same CA1 pyramidal cell". British Journal of Pharmacology . 142 (2): 317–322. doi:10.1038/sj.bjp.0705744. PMC 1574942 . PMID 15155538.
- 1 2 3 Komai, Shoji; Matsuyama, Tomohiro; Matsumoto, Kazumasa; Kato, Keiko; Kobayashi, Masayuki; Imamura, Kazuyuki; Yoshida, Shigetaka; Ugawa, Shinya; Shiosaka, Sadao (April 2000). "Neuropsin regulates an early phase of Schaffer-collateral long-term potentiation in the murine hippocampus". European Journal of Neuroscience. 12 (4): 1479–1486. doi:10.1046/j.1460-9568.2000.00035.x. PMID 10762375. S2CID 44257092.
- 1 2 Lin, Mike T; Luján, Rafael; Watanabe, Masahiko; Adelman, John P; Maylie, James (20 January 2008). "SK2 channel plasticity contributes to LTP at Schaffer collateral–CA1 synapses". Nature Neuroscience. 11 (2): 170–177. doi:10.1038/nn2041. PMC 2613806 . PMID 18204442.
- ↑ Hoffman, D. A.; Sprengel, R.; Sakmann, B. (21 May 2002). "Molecular dissection of hippocampal theta-burst pairing potentiation". Proceedings of the National Academy of Sciences. 99 (11): 7740–7745. Bibcode:2002PNAS...99.7740H. doi: 10.1073/pnas.092157999 . PMC 124338 . PMID 12032353.
- ↑ Meighan, Peter C.; Meighan, Starla E.; Davis, Christopher J.; Wright, John W.; Harding, Joseph W. (September 2007). "Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses". Journal of Neurochemistry. 102 (6): 2085–2096. doi:10.1111/j.1471-4159.2007.04682.x. PMID 17587312. S2CID 3753928.
- ↑ Stanton, Patric K.; Winterer, Jochen; Zhang, Xiao-lei; Müller, Wolfgang (November 2005). "Imaging LTP of presynaptic release of FM1-43 from the rapidly recycling vesicle pool of Schaffer collateral-CA1 synapses in rat hippocampal slices". European Journal of Neuroscience. 22 (10): 2451–2461. doi:10.1111/j.1460-9568.2005.04437.x. PMID 16307588. S2CID 9359440.
- ↑ Christie, J. M.; Jahr, CE (4 January 2006). "Multivesicular release at Schaffer collateral-CA1 hippocampal synapses". Journal of Neuroscience. 26 (1): 210–216. doi:10.1523/JNEUROSCI.4307-05.2006. PMC 2670931 . PMID 16399689.