
The entorhinal cortex (EC) is an area of the brain's allocortex, located in the medial temporal lobe, whose functions include being a widespread network hub for memory, navigation, and the perception of time. The EC is the main interface between the hippocampus and neocortex. The EC-hippocampus system plays an important role in declarative (autobiographical/episodic/semantic) memories and in particular spatial memories including memory formation, memory consolidation, and memory optimization in sleep. The EC is also responsible for the pre-processing (familiarity) of the input signals in the reflex nictitating membrane response of classical trace conditioning; the association of impulses from the eye and the ear occurs in the entorhinal cortex.

The hippocampus, also hippocampus proper, is a major component of the brain of humans and many other vertebrates. In the human brain the hippocampus, the dentate gyrus, and the subiculum are the components of the hippocampal formation located in the limbic system. The hippocampus plays important roles in the consolidation of information from short-term memory to long-term memory, and in spatial memory that enables navigation. In humans, and other primates the hippocampus is located in the archicortex, one of the three regions of allocortex, in each hemisphere with neural projections to the neocortex. The hippocampus, as the medial pallium, is a structure found in all vertebrates.
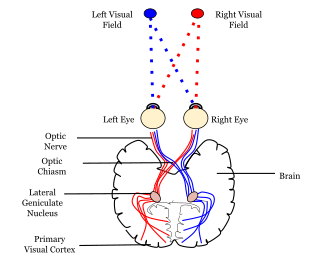
In neuroanatomy, a neural pathway is the connection formed by axons that project from neurons to make synapses onto neurons in another location, to enable neurotransmission. Neurons are connected by a single axon, or by a bundle of axons known as a nerve tract, or fasciculus. Shorter neural pathways are found within grey matter in the brain, whereas longer projections, made up of myelinated axons, constitute white matter.

A place cell is a kind of pyramidal neuron in the hippocampus that becomes active when an animal enters a particular place in its environment, which is known as the place field. Place cells are thought to act collectively as a cognitive representation of a specific location in space, known as a cognitive map. Place cells work with other types of neurons in the hippocampus and surrounding regions to perform this kind of spatial processing. They have been found in a variety of animals, including rodents, bats, monkeys and humans.
Schaffer collaterals are axon collaterals given off by CA3 pyramidal cells in the hippocampus. These collaterals project to area CA1 of the hippocampus and are an integral part of memory formation and the emotional network of the Papez circuit, and of the hippocampal trisynaptic loop. It is one of the most studied synapses in the world and named after the Hungarian anatomist-neurologist Károly Schaffer.
An apical dendrite is a dendrite that emerges from the apex of a pyramidal cell. Apical dendrites are one of two primary categories of dendrites, and they distinguish the pyramidal cells from spiny stellate cells in the cortices. Pyramidal cells are found in the prefrontal cortex, the hippocampus, the entorhinal cortex, the olfactory cortex, and other areas. Dendrite arbors formed by apical dendrites are the means by which synaptic inputs into a cell are integrated. The apical dendrites in these regions contribute significantly to memory, learning, and sensory associations by modulating the excitatory and inhibitory signals received by the pyramidal cells.

Hippocampal sclerosis (HS) or mesial temporal sclerosis (MTS) is a neuropathological condition with severe neuronal cell loss and gliosis in the hippocampus. Neuroimaging tests such as magnetic resonance imaging (MRI) and positron emission tomography (PET) may identify individuals with hippocampal sclerosis. Hippocampal sclerosis occurs in 3 distinct settings: mesial temporal lobe epilepsy, adult neurodegenerative disease and acute brain injury.
Head direction (HD) cells are neurons found in a number of brain regions that increase their firing rates above baseline levels only when the animal's head points in a specific direction. They have been reported in rats, monkeys, mice, chinchillas and bats, but are thought to be common to all mammals, perhaps all vertebrates and perhaps even some invertebrates, and to underlie the "sense of direction". When the animal's head is facing in the cell's "preferred firing direction" these neurons fire at a steady rate, but firing decreases back to baseline rates as the animal's head turns away from the preferred direction.

The hippocampal formation is a compound structure in the medial temporal lobe of the brain. It forms a c-shaped bulge on the floor of the temporal horn of the lateral ventricle. There is no consensus concerning which brain regions are encompassed by the term, with some authors defining it as the dentate gyrus, the hippocampus proper and the subiculum; and others including also the presubiculum, parasubiculum, and entorhinal cortex. The hippocampal formation is thought to play a role in memory, spatial navigation and control of attention. The neural layout and pathways within the hippocampal formation are very similar in all mammals.
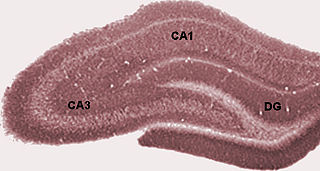
In the brain, the perforant path or perforant pathway provides a connectional route from the entorhinal cortex to all fields of the hippocampal formation, including the dentate gyrus, all CA fields, and the subiculum.

A grid cell is a type of neuron within the entorhinal cortex that fires at regular intervals as an animal navigates an open area, allowing it to understand its position in space by storing and integrating information about location, distance, and direction. Grid cells have been found in many animals, including rats, mice, bats, monkeys, and humans.
The perirhinal cortex is a cortical region in the medial temporal lobe that is made up of Brodmann areas 35 and 36. It receives highly processed sensory information from all sensory regions, and is generally accepted to be an important region for memory. It is bordered caudally by postrhinal cortex or parahippocampal cortex and ventrally and medially by entorhinal cortex.

In the hippocampus, the mossy fiber pathway consists of unmyelinated axons projecting from granule cells in the dentate gyrus that terminate on modulatory hilar mossy cells and in Cornu Ammonis area 3 (CA3), a region involved in encoding short-term memory. These axons were first described as mossy fibers by Santiago Ramón y Cajal as they displayed varicosities along their lengths that gave them a mossy appearance.
In the rodent, the parasubiculum is a retrohippocampal isocortical structure, and a major component of the subicular complex. It receives numerous subcortical and cortical inputs, and sends major projections to the superficial layers of the entorhinal cortex.
The trisynaptic circuit or trisynaptic loop is a relay of synaptic transmission in the hippocampus. The trisynaptic circuit is a neural circuit in the hippocampus, which is made up of three major cell groups: granule cells in the dentate gyrus, pyramidal neurons in CA3, and pyramidal neurons in CA1. The hippocampal relay involves 3 main regions within the hippocampus which are classified according to their cell type and projection fibers. The first projection of the hippocampus occurs between the entorhinal cortex (EC) and the dentate gyrus (DG). The entorhinal cortex transmits its signals from the parahippocampal gyrus to the dentate gyrus via granule cell fibers known collectively as the perforant path. The dentate gyrus then synapses on pyramidal cells in CA3 via mossy cell fibers. CA3 then fires to CA1 via Schaffer collaterals which synapse in the subiculum and are carried out through the fornix. Collectively the dentate gyrus, CA1 and CA3 of the hippocampus compose the trisynaptic loop.

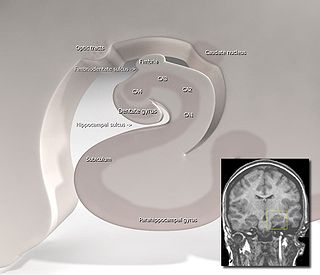
Hippocampus anatomy describes the physical aspects and properties of the hippocampus, a neural structure in the medial temporal lobe of the brain. It has a distinctive, curved shape that has been likened to the sea-horse monster of Greek mythology and the ram's horns of Amun in Egyptian mythology. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. For example, in the rat, the two hippocampi look similar to a pair of bananas, joined at the stems. In the human and other primates, the portion of the hippocampus near the base of the temporal lobe is much broader than the part at the top. Due to the three-dimensional curvature of this structure, two-dimensional sections such as shown are commonly seen. Neuroimaging pictures can show a number of different shapes, depending on the angle and location of the cut.

Boundary cells are neurons found in the hippocampal formation that respond to the presence of an environmental boundary at a particular distance and direction from an animal. The existence of cells with these firing characteristics were first predicted on the basis of properties of place cells. Boundary cells were subsequently discovered in several regions of the hippocampal formation: the subiculum, presubiculum and entorhinal cortex.

Sharp waves and ripples (SPW-R), also called sharp wave ripples (SWR), are oscillatory patterns produced by extremely synchronized activity of neurons in the mammalian hippocampus and neighboring regions which occur spontaneously in idle waking states or during NREM sleep. They can be observed with a variety of electrophysiological methods such as field recordings or EEG. They are composed of large amplitude sharp waves in local field potential and produced by thousands of neurons firing together within a 30–100 ms window. Within this broad time window, pyramidal cells fire only at specific times set by fast spiking GABAergic interneurons. The fast rhythm of inhibition synchronizes the firing of active pyramidal cells, each of which only fires one or two action potentials exactly between the inhibitory peaks, collectively generating the ripple pattern. SWRs have been extensively characterized by György Buzsáki and have been shown to be involved in memory consolidation in NREM sleep. Neuronal firing sequences acquired during wakefulness are replayed during SWRs.
The hippocampus participates in the encoding, consolidation, and retrieval of memories. The hippocampus is located in the medial temporal lobe (subcortical), and is an infolding of the medial temporal cortex. The hippocampus plays an important role in the transfer of information from short-term memory to long-term memory during encoding and retrieval stages. These stages do not need to occur successively, but are, as studies seem to indicate, and they are broadly divided in the neuronal mechanisms that they require or even in the hippocampal areas that they seem to activate. According to Gazzaniga, "encoding is the processing of incoming information that creates memory traces to be stored." There are two steps to the encoding process: "acquisition" and "consolidation". During the acquisition process, stimuli are committed to short term memory. Then, consolidation is where the hippocampus along with other cortical structures stabilize an object within long term memory, which strengthens over time, and is a process for which a number of theories have arisen to explain the underlying mechanism. After encoding, the hippocampus is capable of going through the retrieval process. The retrieval process consists of accessing stored information; this allows learned behaviors to experience conscious depiction and execution. Encoding and retrieval are both affected by neurodegenerative and anxiety disorders and epilepsy.
The hippocampal subfields are four subfields CA1, CA2, CA3, and CA4 that make up the structure of the hippocampus. Regions described in the hippocampus are the head, body, and tail, and other hippocampal subfields include the dentate gyrus, the presubiculum, and the subiculum. The CA subfields use the initials of cornu ammonis, an earlier name of the hippocampus.














