Related Research Articles

Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs, which may be supplied only to consumers possessing a valid prescription. In many countries, OTC drugs are selected by a regulatory agency to ensure that they contain ingredients that are safe and effective when used without a physician's care. OTC drugs are usually regulated according to their active pharmaceutical ingredient (API) and strengths of final products.

Cold medicines are a group of medications taken individually or in combination as a treatment for the symptoms of the common cold and similar conditions of the upper respiratory tract. The term encompasses a broad array of drugs, including analgesics, antihistamines and decongestants, among many others. It also includes drugs which are marketed as cough suppressants or antitussives, but their effectiveness in reducing cough symptoms is unclear or minimal.
H1 antagonists, also called H1 blockers, are a class of medications that block the action of histamine at the H1 receptor, helping to relieve allergic reactions. Agents where the main therapeutic effect is mediated by negative modulation of histamine receptors are termed antihistamines; other agents may have antihistaminergic action but are not true antihistamines.
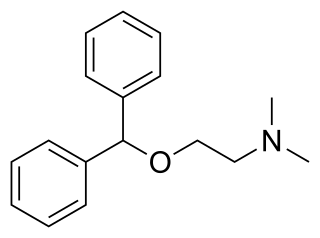
Diphenhydramine, sold under the brand name Benadryl among others, is an antihistamine and sedative. It is a first-generation H1-antihistamine and it works by blocking certain effects of histamine, which produces its antihistamine and sedative effects. Diphenhydramine is also a potent anticholinergic. It is mainly used to treat allergies, insomnia, and symptoms of the common cold. It is also less commonly used for tremors in parkinsonism, and nausea. It is taken by mouth, injected into a vein, injected into a muscle, or applied to the skin. Maximal effect is typically around two hours after a dose, and effects can last for up to seven hours.

Loratadine, sold under the brand name Claritin among others, is a medication used to treat allergies. This includes allergic rhinitis and hives. It is also available in drug combinations such as loratadine/pseudoephedrine, in which it is combined with pseudoephedrine, a nasal decongestant. It is taken orally.

Benadryl is a brand of various antihistamine medications used to stop allergies, whose content varies in different countries, but which includes some combination of diphenhydramine, acrivastine, and/or cetirizine.
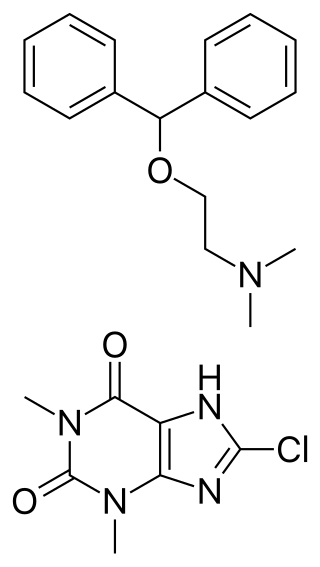
Dimenhydrinate, also known as diphenhydramine/8-chlorotheophylline salt and sold under the brand name Dramamine, Gravol, among others, is an over-the-counter medication used to treat motion sickness and nausea. Dimenhydrinate is a theoclate salt composed of diphenhydramine and 8-chlorotheophylline in a 1:1 ratio.

Hydroxyzine, sold under the brand names Atarax and Vistaril among others, is an antihistamine medication. It is used in the treatment of itchiness, anxiety, insomnia, and nausea. It is used either by mouth or injection into a muscle.
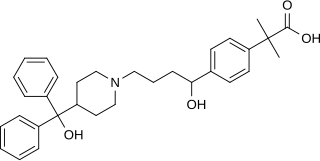
Fexofenadine, sold under the brand name Allegra among others, is an antihistamine pharmaceutical drug used in the treatment of allergy symptoms, such as hay fever and urticaria.

Promethazine, sold under the brand name Phenergan among others, is a first-generation antihistamine, sedative, and antiemetic used to treat allergies, insomnia, and nausea. It may also help with some symptoms associated with the common cold and may also be used for sedating people who are agitated or anxious, an effect that has led to some recreational use. Promethazine is taken by mouth (oral), as a rectal suppository, or by injection into a muscle (IM).

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.

Phenylephrine, sold under the brand names Neosynephrine and Sudafed PE among others, is a medication used as a decongestant for uncomplicated nasal congestion in the form of a nasal spray or oral tablet, to dilate the pupil, to increase blood pressure given intravenously in cases of low blood pressure, and to relieve hemorrhoids as a suppository. It can also be applied to the skin.
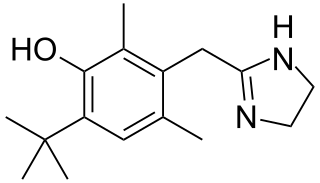
Oxymetazoline, sold under the brand name Afrin among others, is a topical decongestant and vasoconstrictor medication. It is available over-the-counter as a nasal spray to treat nasal congestion and nosebleeds, as eye drops to treat eye redness due to minor irritation, and as a prescription topical cream to treat persistent facial redness due to rosacea in adults. Its effects begin within minutes and last for up to six hours. Intranasal use for longer than three to five days may cause congestion to recur or worsen, resulting in physical dependence.

Doxylamine is an antihistamine medication used to treat insomnia and allergies, and—in combination with pyridoxine (vitamin B6)—to treat morning sickness in pregnant women. It is available over-the-counter and is typically sold under such brand names as Equate or Unisom, among others; and it is used in nighttime cold medicines (e.g., NyQuil) and pain medications containing acetaminophen and/or codeine to help with sleep. The medication is delivered chemically by the salt doxylamine succinate and is taken by mouth. Doxylamine and other first-generation antihistamines are the most widely used sleep medications in the world. Typical side effects of doxylamine (at recommended doses) include dizziness, drowsiness, grogginess, and dry mouth, among others.

Doxepin is a medication belonging to the tricyclic antidepressant (TCA) class of drugs used to treat major depressive disorder, anxiety disorders, chronic hives, and insomnia. For hives it is a less preferred alternative to antihistamines. It has a mild to moderate benefit for sleeping problems. It is used as a cream for itchiness due to atopic dermatitis or lichen simplex chronicus.

Orphenadrine is an anticholinergic drug of the ethanolamine antihistamine class; it is closely related to diphenhydramine. It is a muscle relaxant that is used to treat muscle pain and to help with motor control in Parkinson's disease, but has largely been superseded by newer drugs. It is considered a dirty drug due to its multiple mechanisms of action in different pathways. It was discovered and developed in the 1940s.
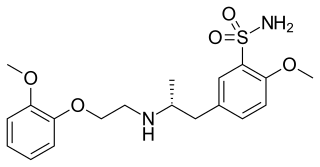
Tamsulosin, sold under the brand name Flomax among others, is a medication used to treat symptomatic benign prostatic hyperplasia (BPH) and chronic prostatitis and to help with the passage of kidney stones. The evidence for benefit with a kidney stone is better when the stone is larger. Tamsulosin is taken by mouth.

Methapyrilene is an antihistamine and anticholinergic of the pyridine chemical class which was developed in the early 1950s. It was sold under the trade names Co-Pyronil and Histadyl EC in the UK. It has relatively strong sedative effects, to the extent that its primary use was as a medication for insomnia rather than for its antihistamine action. Together with scopolamine, it was the main ingredient in Sominex, Nytol, and Sleep-Eze. It also provided the sedative component of Excedrin PM. All of these products were reformulated in the late 1970s when methapyrilene was demonstrated to cause liver cancer in rats when given chronically.
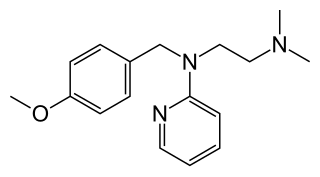
Mepyramine, also known as pyrilamine, is a first-generation antihistamine, targeting the H1 receptor as an inverse agonist. Mepyramine rapidly permeates the brain, often causing drowsiness. It is often sold as a maleate salt, pyrilamine maleate.

Bromazine, sold under the brand names Ambodryl, Ambrodil, and Deserol among others, also known as bromodiphenhydramine, is an antihistamine and anticholinergic medication of the ethanolamine class. It is an analogue of diphenhydramine with a bromine substitution on one of the phenyl rings.
References
- ↑ Ranii, David (21 December 2011). "GSK sells BC, Goody's and other brands". News & Observer . Archived from the original on 15 April 2012.
- ↑ "SOMINEX- diphenhydramine hydrochloride tablet, film coated". DailyMed . Retrieved 16 April 2024– via National Library of Medicine.
- ↑ "Mother To Baby | Fact Sheets [Internet]: Diphenhydramine". National Library of Medicine . January 2023. Retrieved 16 April 2024.
- ↑ "SOMINEX- diphenhydramine hydrochloride tablet". DailyMed . Retrieved 16 April 2024– via FDA.
- ↑ "Elimination Guidelines (April 3, 2023)". Government of Canada. Retrieved 16 April 2024.
- ↑ Csiernik, Rick (2009). The Essential Guide to Psychoactive Drugs in Canada: A Resource for Counselling Professionals (2nd ed.). Ontario: Canadian Scholars. p. 82. ISBN 9781773381602.
- ↑ "Sominex" (PDF). Medicines.org UK. Retrieved 16 April 2024.
- 1 2 "Sominex Tablets". Medicines.org UK. Retrieved 16 April 2024.
- ↑ "Lunar Biopharma". lunarbio.com. Archived from the original on 13 October 2012. Retrieved 17 January 2022.
- ↑ "Diphenhydramine Hydrochloride (Sominex)". GNH India. Retrieved 16 April 2024.
- ↑ "Diphenhydramine: drowsy (sedating) antihistamine". National Health Service . 21 September 2018. Retrieved 1 November 2023.
- ↑ "Label: TYLENOL PM EXTRA STRENGTH- acetaminophen and diphenhydramine hydrochloride tablet, film coated". DailyMed . Retrieved 16 April 2024– via National Library of Medicine.
- ↑
- "Promethazine (Phenergan)". National Health Service . Retrieved 16 April 2024.
- Southard, Brittin T.; Al Khalili, Yasir (1 January 2024). "Promethazine". StatPearls [Internet]. Retrieved 16 April 2024– via National Library of Medicine.
- ↑ Sominex commercial 1958 (Commercial). Sominex. 21 May 2012. Archived from the original on 9 August 2013. Retrieved 14 April 2018– via YouTube.
- ↑ Sominex commercial on the Arthur Murray Dance Party 1958 (Commercial). NBC (published 18 May 2020). 1958. Retrieved 15 April 2024– via YouTube.
- ↑ Anderson, Jack (3 November 1959). "Man Behind the Medicine". The Post Register, Idaho Falls . Vol. 29, no. 3. p. 4 – via NewspaperArchive.com.
- ↑ Anderson, Jack (3 November 1959). "Quiz Boosts Sales". The Post Register, Idaho Falls . Vol. 29, no. 3. p. 4 – via NewspaperArchive.com.
- ↑ "New Way to End Sleepless Nights ...Safely*Taken as directed" . New York Daily News . Vol. 1, no. 257. 20 April 1960. p. 19 – via Newspapers.com.
- 1 2 Fass, Grace (December 1971). "Sleep, Drugs, and Dreams". American Journal of Nursing . 71 (12): 2316–2320. doi:10.2307/3422235. JSTOR 3422235. PMID 5209451. S2CID 22422200.
- ↑ Weil, Henry (13 February 1977). "Do your sleeping pills really work?" . The Daily News. pp. 8, 9, 16 – via Newspapers.com.
- ↑ Montgomery, Jerry (28 June 1978). "Sleeping aids − What's in those tablets?" . The Santa Fe New Mexican . Vol. 129, no. 194. Gannett. p. 10 – via Newspapers.com.
- 1 2 3 "FDA Orders Sominex 2 Withdrawn From Market". Richmond Times-Dispatch . Vol. 125, no. 336. 2 December 1975. p. 2. Retrieved 16 April 2024– via Newspapers.com.
- ↑ Morrant, J. C. A. (June 1975). "Medicines and Mental Illness in Old Age". Canadian Psychiatric Association Journal . 20 (4): 247–324. doi: 10.1177/070674377502000412 . ISSN 0008-4824. PMID 1182644. S2CID 27695558.
- ↑ "Few Beers Before Test?". Barstow Desert Dispatch . Vol. 64, no. 298. 9 April 1977. p. 3 – via NewspaperArchive.com.
- ↑ National Research Council (US) Panel on Anticholinergic Chemicals (1982). Possible Long-Term Health Effects of Short-Term Exposure to Chemical Agents: Volume 1 Anticholinesterases and Anticholinergics (Report). Washington D.C.: National Academies Press. p. Appendix B. Retrieved 16 April 2024– via National Library of Medicine.
- 1 2 Stewart, Ronald B.; May, Franklin E.; Moore, Mary T.; Hale, William E. (August 1989). "Changing Patterns of Psychotropic Drug use in the Elderly: A Five-Year Update". DICP: The Annals of Pharmacotherapy . 23 (7–8): 610–613. doi:10.1177/1060028089023007-821. ISSN 1042-9611. PMID 2569792. S2CID 33206308. See table 2.
- ↑ "Sleep aids testing beefed up" . Racine Journal Times . Vol. 122, no. 162. 13 June 1978. p. 21 – via Newspapers.com.
- ↑ Lijinsky, W.; Reuber, M.D.; Blackwell, B.N. (August 1980). "Liver tumors induced in rats by oral administration of the antihistaminic methapyrilene hydrochloride". Science . 209 (4458): 817–9. Bibcode:1980Sci...209..817L. doi:10.1126/science.7403848. PMID 7403848.
- ↑ "FDA Plans Ban Of Sleeping Pills" . Florida Today . Vol. 13, no. 84. 13 June 1978. p. 11A – via Newspapers.com.
- ↑ "Many sleep aids expected to be off market by June" . The Modesto Bee . Vol. 102, no. 127. 7 May 1979. p. 7 – via Newspapers.com.
- ↑ "FDA Seeks Restrictions On Sleeping Aid Drugs" . Daily Press . Vol. 83, no. 164. 13 June 1978. p. 1 – via Newspapers.com.
- ↑ Cook, Christopher (28 June 1979). "Sleep aids back with new drug | Critics assail 'human testing'" . The Minneapolis Star . pp. 1A, 6A – via Newspapers.com.
- 1 2 "FDA approves most sleep-aid ingredients" . Arizona Daily Star . Vol. 148, no. 45. 14 February 1989. p. 6 – via Newspapers.com.
- 1 2 54 FR 6826
- ↑ Rausch, Tim (14 April 2011). "Drugmaker GlaxoSmithKline to sell Aiken facility". The Augusta Chronicle . Associated Press . Retrieved 16 April 2024.
- ↑
- Amato, Michael (18 January 2012). "Prestige Brands Sets Rate on $620 Million Loan for Buyout" . Bloomberg News . Retrieved 16 April 2024.
- "GlaxoSmithKline Sells North American Brands to Prestige Brands, Inc. for $661.6 Million Cash". BioSpace (Press release). 20 December 2011. Retrieved 16 April 2024.
- 1 2 Nocera, Joseph (17 December 1975). "No Chance This Year for Consumer Bill" . Garden City Telegram . Vol. 47, no. 39. p. 24 – via Newspapers.com.
- 1 2 "Sominex Faces Suit by State" . Oroville Mercury-Register . Vol. 101, no. 277. Sacramento. 25 November 1975. p. 2 – via Newspapers.com.
- ↑ "Recall" . The Herald-News. Vol. 104, no. 307. 29 December 1975. p. 15 – via Newspapers.com.
- ↑ Graedon, Joe (5 December 1983). "Sleeping-ill ingredient taken from allergy drug" . Springfield News-Leader . p. 4B – via Newspapers.com.
- ↑ "What is Sominex? | Ingredients". Sominex Sleep UK. Archived from the original on 3 March 2011. Retrieved 16 April 2024.
- 1 2 Adam, Kirstine; Oswald, I. (December 1986). "The hypnotic effects of an antihistamine: promethazine". British Journal of Clinical Pharmacology . 22 (6): 715–717. doi: 10.1111/j.1365-2125.1986.tb02962.x . PMC 1401211 . PMID 3567016. S2CID 35326486.
- ↑ "What is Sominex Herbal? | Ingredients". Sominex Sleep UK. Archived from the original on 3 March 2011. Retrieved 16 April 2024.
- ↑ "Sominex counts on sheep for growth" (PDF). OTC Bulletin. 13 August 2010. p. 17. Retrieved 16 April 2024.
- ↑ "Human Medicines Withdrawn Products" (PDF). Department of Health (Ireland) . Retrieved 16 April 2024.
- ↑ Restrictions in Use and Availability of Pharmaceuticals, 2010-2018 (Report). Geneva: World Health Organization. 1 January 2020. p. 164. ISBN 978-92-4-001477-0. JSTOR resrep48218.4.
- ↑ Stein, Richard A.; Strickland, Tony L. (April 1998). "A Review of the Neuropsychological Effects of Commonly Used Prescription Medications". Archives of Clinical Neuropsychology. 13 (3): 259–284. doi: 10.1016/S0887-6177(97)00027-9 . PMID 14590642. S2CID 23375547.
- ↑ Schifano, Fabrizio; Chiappini, Stefania; Miuli, Andrea; et al. (7 May 2021). "Focus on Over-the-Counter Drugs' Misuse: A Systematic Review on Antihistamines, Cough Medicines, and Decongestants". Frontiers in Psychiatry . 12: 657397. doi: 10.3389/fpsyt.2021.657397 . PMC 8138162 . PMID 34025478. S2CID 233874257. See table 1.
- 1 2 Claiborne, William (1 October 1969). "Over-the-Counter Sleeping Pill's Side Effects Suspected" . Courier News . Vol. 86, no. 89. p. 23 – via Newspapers.com.
- ↑ Fatteh, Abdullah; Dudley, J.B. (7 February 1972). "Fatal Poisoning Involving Methapyrilene". JAMA . 219 (6): 756–757. doi:10.1001/JAMA.1972.03190320056027. PMID 4400422. S2CID 5329969.
- 1 2 Klotz, Neil (24 October 1975). "Finding Obtained From FDA: Are Pills And Tranquilizers Worth It?". Westminster College Parson. Vol. 1, no. 8. p. 9 – via Newspapers.com.
- ↑ "Scientists report money wasted on sleeping pills and sedatives" . The Daily Mail. Vol. 147, no. 287. Bridgerstown, MD. 6 December 1975. p. 6 – via Newspapers.com.
- ↑ Micik, Sylvia (January 1976). "Anticholinergic Poisoning—New Antidote". Western Journal of Medicine . 124 (1): 50–51. PMC 129975 . PMID 18747630. S2CID 38789858.
- ↑ Mintzer, Jacobo; Burns, Alistair (September 2000). "Anticholinergic side-effects of drugs in elderly people". Journal of the Royal Society of Medicine . 93 (9): 457–462. doi: 10.1177/014107680009300903 . PMC 1298101 . PMID 11089480. S2CID 43591230.
- ↑ Jones, B; Lal, S (August 1985). "Tardive dyskinesia uncovered after ingestion of Sominex, an over-the-counter drug". The Canadian Journal of Psychiatry . 30 (5): 370–371. doi:10.1177/070674378503000514. eISSN 1497-0015. PMID 2862985. S2CID 37797680.
- ↑ Bogenschutz, Michael P.; Ross, Stephen; Bhatt, Snehal; et al. (24 August 2022). "Percentage of Heavy Drinking Days Following Psilocybin-Assisted Psychotherapy vs Placebo in the Treatment of Adult Patients With Alcohol Use Disorder". JAMA Psychiatry . 79 (10): 953–962. doi: 10.1001/jamapsychiatry.2022.2096 . PMC 9403854 . PMID 36001306. S2CID 251766399.
- ↑ Carr, Teresa (12 December 2018). "The Problem With Sleeping Pills". Consumer Reports . Retrieved 16 April 2024.
- ↑ Graedon, Joe (19 August 1979). "Sleeping Pill Reports Confirmed" . Alexandria Daily Town Talk. Vol. 97, no. 156. p. E-7 – via Newspapers.com.
- ↑ Kirkman, Don (22 July 1971). "Stricter Control of Drugs to Be Asked". Knoxville News Sentinel . No. 29, 075. p. 24. Retrieved 16 April 2024– via Newspapers.com.
- ↑ Anderson, Jack (17 October 1975). "Washington merry-go-round | Public service profitable for senator". Delphos Tri County Daily Herald. Vol. 106, no. 106. p. 6 – via NewspaperArchive.com.
- ↑ Royer, Fred L. (Winter 1972). "State of the Science Report: Effects of Psychoactive Drugs on Behavioral Functions". Journal of Drug Issues . 2 (1): 29–33. doi:10.1177/002204267200200106. ISSN 0022-0426. S2CID 80052408.
- ↑ Choate, Robert; Debevoise, Nancy (January 1976). "Caution! Keep This Commercial Out of Reach of Children!". Journal of Drug Issues . 6 (1): 91–98. doi:10.1177/002204267600600117. ISSN 0022-0426. S2CID 80315622.
- ↑ Feldman, Marc D.; Behar, Marcy (13 June 1986). "A Case of Massive Diphenhydramine Abuse and Withdrawal From Use of the Drug". JAMA . 55 (22): 3119–3120. doi:10.1001/jama.1986.03370220081028. PMID 3702020. S2CID 20105532.
- ↑ Barlow, Julie; Charlton, Bruce G. (9 September 2005). "Letter to the Editor | Self-management and pregnancy–safe interventions for panic, phobia and other anxiety-disorders might include over-the-counter (OTC) 'SSRI' antihistamines such as diphenhydramine and chlorpheniramine". Acta Psychiatrica Scandinavica . 112 (3): 323. doi:10.1111/j.1600-0447.2005.00611.x. PMID 16156843. S2CID 19105124.
- ↑ Soper, John; Cliburn, Kacey; Hileman, Christy; Craft, Kristi; Kemp, Philip (31 December 2020). Comparative Evaluation of Forensic Toxicology Findings, 2012-2016 (PDF) (Report). Federal Aviation Administration. pp. 9–10. S2CID 265537577 . Retrieved 17 April 2024.
- ↑ Sansgiry, Sujit S.; Bhansali, Archita H.; Bapat, Shweta S.; Zu, Qingqing (19 December 2016). "Abuse of over-the-counter medicines: a pharmacist's perspective". Integrated Pharmacy Research and Practice . 6 (2017): 1–6. doi: 10.2147/IPRP.S103494 . PMC 5774309 . PMID 29354545. S2CID 12160975. See tables 2 & 3.
- ↑ Pal, Meera (22 September 2023). Smith, Kelly Anne (ed.). "Benadryl For Dogs: Safe Dosages And Uses". Forbes . Retrieved 16 April 2024.
- ↑ Zornosa, Laura (18 October 2023). "The True Story Behind the Netflix Documentary The Devil on Trial". TIME . Retrieved 25 March 2024.
- ↑ Lloyd, Sophie (18 October 2023). "'Devil on Trial' Shock Reveal—Can Sleeping Pills Really Mimic Possession?". Newsweek . Retrieved 31 March 2024.
- 1 2 Oyekan, Princess J.; Gorton, Hayley C.; Copeland, Caroline S. (17 March 2021). "Antihistamine-related deaths in England: Are the high safety profiles of antihistamines leading to their unsafe use?". British Journal of Clinical Pharmacology . 87 (10): 3978–3987. doi: 10.1111/bcp.14819 . PMID 33729599. S2CID 232262504.