
Sponges, the members of the phylum Porifera, are a basal animal clade as a sister of the diploblasts. They are multicellular organisms that have bodies full of pores and channels allowing water to circulate through them, consisting of jelly-like mesohyl sandwiched between two thin layers of cells.
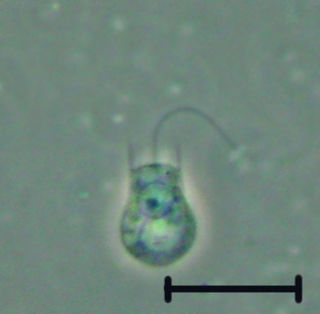
The choanoflagellates are a group of free-living unicellular and colonial flagellate eukaryotes considered to be the closest living relatives of the animals. Choanoflagellates are collared flagellates, having a funnel shaped collar of interconnected microvilli at the base of a flagellum. Choanoflagellates are capable of both asexual and sexual reproduction. They have a distinctive cell morphology characterized by an ovoid or spherical cell body 3–10 µm in diameter with a single apical flagellum surrounded by a collar of 30–40 microvilli. Movement of the flagellum creates water currents that can propel free-swimming choanoflagellates through the water column and trap bacteria and detritus against the collar of microvilli, where these foodstuffs are engulfed. This feeding provides a critical link within the global carbon cycle, linking trophic levels. In addition to their critical ecological roles, choanoflagellates are of particular interest to evolutionary biologists studying the origins of multicellularity in animals. As the closest living relatives of animals, choanoflagellates serve as a useful model for reconstructions of the last unicellular ancestor of animals.

Demosponges (Demospongiae) are the most diverse class in the phylum Porifera. They include 76.2% of all species of sponges with nearly 8,800 species worldwide. They are sponges with a soft body that covers a hard, often massive skeleton made of calcium carbonate, either aragonite or calcite. They are predominantly leuconoid in structure. Their "skeletons" are made of spicules consisting of fibers of the protein spongin, the mineral silica, or both. Where spicules of silica are present, they have a different shape from those in the otherwise similar glass sponges. Some species, in particular from the Antarctic, obtain the silica for spicule building from the ingestion of siliceous diatoms.

Grantia is a genus of calcareous sponges belonging to the family Grantiidae. Species of the genus Grantia contain spicules and spongin fibers.

Suberites domuncula is a species of sea sponge belonging to the family Suberitidae.

Polymastia is a genus of sea sponges containing about 30 species. These are small to large encrusting or dome-shaped sponges with a smooth surface having many teat-shaped projections (papillae). In areas of strong wave action, this genus does not grow the teat structures, but instead grows in a corrugated form.

Chondrocladia is a genus of carnivorous demosponges of the family Cladorhizidae. Neocladia was long considered a junior synonym, but has recently become accepted as a distinct genus.

Axinella is a genus of sponges in the family Axinellidae first described in 1862 by Eduard Oscar Schmidt. Species of Axinella occur in the Indian and Pacific Oceans. Most of these sponges are smaller than 20 cm, and have a yellow or orange colour.

Spicules are structural elements found in most sponges. The meshing of many spicules serves as the sponge's skeleton and thus it provides structural support and potentially defense against predators.
Monorhaphis is a monotypic genus of siliceous deep sea Hexactinellid sponges. The single species is the type species Monorhaphis chuni, a sponge known for creating a single giant basal spicule (G.B.S.) to anchor the sponge in the sediments. The species was described by Franz Eilhard Schulze in 1904 from specimens collected by the German Deep Sea Expedition in 1898-1899. Monorhaphis is also the only genus in the monotypic family Monorhaphididae.
Homaxinella is a genus of sea sponges in the family Suberitidae. The type species is Homaxinella balfourensis.
Homaxinella balfourensis is a species of sea sponge in the family Suberitidae. It is found in the seas around Antarctica and can grow in two forms, either branching out in one plane like a fan or forming an upright club-like structure.

Until the late 1950s, the Precambrian was not believed to have hosted multicellular organisms. However, with radiometric dating techniques, it has been found that fossils initially found in the Ediacara Hills in Southern Australia date back to the late Precambrian. These fossils are body impressions of organisms shaped like disks, fronds and some with ribbon patterns that were most likely tentacles.

Gelliodes is a genus of sponges in the family Niphatidae.
Amphilectus is a genus of demosponges, comprising around 20 species found in oceans around the world.

Silicateins are enzymes which catalyse the formation of biosilica from monomeric silicon compounds extracted from the natural environment. Environmental silicates are absorbed by specific biota, including diatoms, radiolaria, silicoflagellates, and siliceous sponges; silicateins have so far only been found in sponges. Silicateins are homologous to the cysteine protease cathepsin.
Hamacantha is a genus of sponges in the family Hamacanthidae. This species in this genus differ from those in the other genera in this family through the presence of diancistras, distinctive microscleres. These are thought to aid in framing the skeleton by joining monactine megascleres. This genus contains 30 species in three subgenera.

Phorbas is a genus of demosponges belonging to the family Hymedesmiidae.

Latrunculia is a genus of demosponges. It is well known for the diverse array of chemical compounds found in its species, including the latrunculins, which are named after this genus. Many of these are medically important, including anti-cancer compounds such as discorhabdins.
Tetilla is a genus of demosponges in the family Tetillidae. It is widely distributed. They are mainly found in deeper habitats.














