Related Research Articles

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery.

An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells that generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.

A fuel cell is an electrochemical cell that converts the chemical energy of a fuel and an oxidizing agent into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied.

A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other rechargeable batteries, Li-ion batteries are characterized by higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991: within the next 30 years, their volumetric energy density increased threefold while their cost dropped tenfold.
A regenerative fuel cell or reverse fuel cell (RFC) is a fuel cell run in reverse mode, which consumes electricity and chemical B to produce chemical A. By definition, the process of any fuel cell could be reversed. However, a given device is usually optimized for operating in one mode and may not be built in such a way that it can be operated backwards. Standard fuel cells operated backwards generally do not make very efficient systems unless they are purpose-built to do so as with high-pressure electrolysers, regenerative fuel cells, solid-oxide electrolyser cells and unitized regenerative fuel cells.
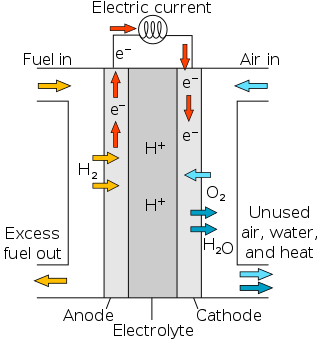
Proton-exchange membrane fuel cells (PEMFC), also known as polymer electrolyte membrane (PEM) fuel cells, are a type of fuel cell being developed mainly for transport applications, as well as for stationary fuel-cell applications and portable fuel-cell applications. Their distinguishing features include lower temperature/pressure ranges and a special proton-conducting polymer electrolyte membrane. PEMFCs generate electricity and operate on the opposite principle to PEM electrolysis, which consumes electricity. They are a leading candidate to replace the aging alkaline fuel-cell technology, which was used in the Space Shuttle.
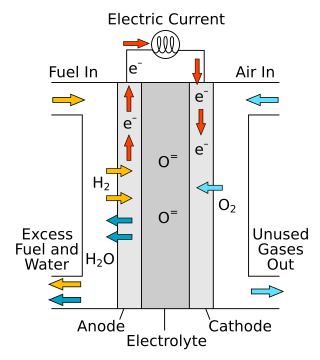
A solid oxide fuel cell is an electrochemical conversion device that produces electricity directly from oxidizing a fuel. Fuel cells are characterized by their electrolyte material; the SOFC has a solid oxide or ceramic electrolyte.

Electrolysis of water is using electricity to split water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture would be extremely explosive. Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can be used for oxyhydrogen welding and other applications, as the hydrogen / oxygen flame can reach approximately 2,800°C.

Aluminium smelting is the process of extracting aluminium from its oxide, alumina, generally by the Hall-Héroult process. Alumina is extracted from the ore bauxite by means of the Bayer process at an alumina refinery.
Genoa Joint Laboratories (GJL) is a scientific research activity founded in 2002, combining expertise in electroceramics and electrochemistry of three facilities: National Research Council - Institute for Energetics and Interphases (CNR-IENI), Department of Chemical and Process Engineering with University of Genova (DICHeP), and the Department of Chemistry and Industrial Chemistry with University of Genova (DCCI), all located in Genoa, Italy.

Yttria-stabilized zirconia (YSZ) is a ceramic in which the cubic crystal structure of zirconium dioxide is made stable at room temperature by an addition of yttrium oxide. These oxides are commonly called "zirconia" (ZrO2) and "yttria" (Y2O3), hence the name.

A lithium-ion capacitor is a hybrid type of capacitor classified as a type of supercapacitor. It is called a hybrid because the anode is the same as those used in lithium-ion batteries and the cathode is the same as those used in supercapacitors. Activated carbon is typically used as the cathode. The anode of the LIC consists of carbon material which is often pre-doped with lithium ions. This pre-doping process lowers the potential of the anode and allows a relatively high output voltage compared to other supercapacitors.

A solid oxide electrolyzer cell (SOEC) is a solid oxide fuel cell that runs in regenerative mode to achieve the electrolysis of water by using a solid oxide, or ceramic, electrolyte to produce hydrogen gas and oxygen. The production of pure hydrogen is compelling because it is a clean fuel that can be stored, making it a potential alternative to batteries, methane, and other energy sources. Electrolysis is currently the most promising method of hydrogen production from water due to high efficiency of conversion and relatively low required energy input when compared to thermochemical and photocatalytic methods.
Nanoarchitectures for lithium-ion batteries are attempts to employ nanotechnology to improve the design of lithium-ion batteries. Research in lithium-ion batteries focuses on improving energy density, power density, safety, durability and cost.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and cost.

Mixed conductors, also known as mixed ion-electron conductors(MIEC), are a single-phase material that has significant conduction ionically and electronically. Due to the mixed conduction, a formally neutral species can transport in a solid and therefore mass storage and redistribution are enabled. Mixed conductors are well known in conjugation with high-temperature superconductivity and are able to capacitate rapid solid-state reactions.
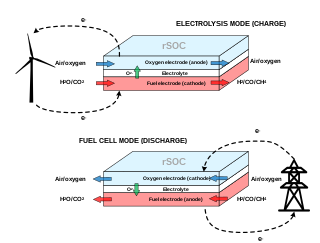
A reversible solid oxide cell (rSOC) is a solid-state electrochemical device that is operated alternatively as a solid oxide fuel cell (SOFC) and a solid oxide electrolysis cell (SOEC). Similarly to SOFCs, rSOCs are made of a dense electrolyte sandwiched between two porous electrodes. Their operating temperature ranges from 600°C to 900°C, hence they benefit from enhanced kinetics of the reactions and increased efficiency with respect to low-temperature electrochemical technologies.
References
- ↑ Fehribach, Joseph D.; O'Hayre, Ryan (January 2009). "Triple Phase Boundaries in Solid-Oxide Cathodes". SIAM Journal on Applied Mathematics. 70 (2): 510–530. doi:10.1137/080722667. ISSN 0036-1399. S2CID 17872247.
- ↑ O’Hayre, Ryan; Prinz, Fritz B. (2004). "The Air/Platinum/Nafion Triple-Phase Boundary: Characteristics, Scaling, and Implications for Fuel Cells". Journal of the Electrochemical Society. 151 (5): A756. Bibcode:2004JElS..151A.756O. doi:10.1149/1.1701868.
- ↑ Vivet, N.; Chupin, S.; Estrade, E.; Richard, A.; Bonnamy, S.; Rochais, D.; Bruneton, E. (December 2011). "Effect of Ni content in SOFC Ni-YSZ cermets: A three-dimensional study by FIB-SEM tomography". Journal of Power Sources. 196 (23): 9989–9997. Bibcode:2011JPS...196.9989V. doi:10.1016/j.jpowsour.2011.07.010.
- ↑ Song, Bowen; Ruiz-Trejo, Enrique; Bertei, Antonio; Brandon, Nigel P. (January 2018). "Quantification of the degradation of Ni-YSZ anodes upon redox cycling". Journal of Power Sources. 374: 61–68. Bibcode:2018JPS...374...61S. doi: 10.1016/j.jpowsour.2017.11.024 . hdl: 10044/1/53328 .
- ↑ Song, B.; Ruiz-Trejo, E.; Brandon, N.P. (August 2018). "Enhanced mechanical stability of Ni-YSZ scaffold demonstrated by nanoindentation and Electrochemical Impedance Spectroscopy". Journal of Power Sources. 395: 205–211. Bibcode:2018JPS...395..205S. doi: 10.1016/j.jpowsour.2018.05.075 . hdl: 10044/1/60309 .
- ↑ Jørgensen, P.S.; Hansen, K.V.; Larsen, R.; Bowen, J.R. (2010-12-15). "High accuracy interface characterization of three phase material systems in three dimensions". Journal of Power Sources. 195 (24): 8168–8176. Bibcode:2010JPS...195.8168J. doi:10.1016/j.jpowsour.2010.06.083.