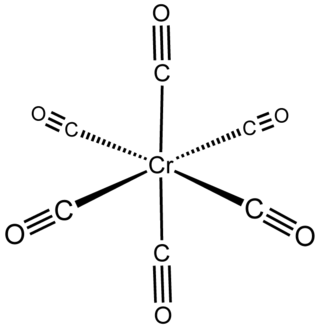
Chromium hexacarbonyl is a chromium(0) organometallic compound with the formula Cr(CO)6. It is a homoleptic complex, which means that all the ligands are identical. It is a colorless crystalline air-stable solid, with a high vapor pressure.
Peter Thomas Wolczanski is the George W. and Grace L. Todd professor of Chemistry at Cornell University.
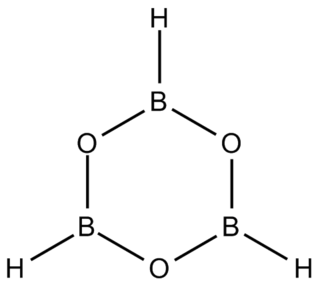
Boroxine is a 6-membered heterocyclic compound composed of alternating oxygen and singly-hydrogenated boron atoms. Boroxine derivatives such as trimethylboroxine and triphenylboroxine also make up a broader class of compounds called boroxines. These compounds are solids that are usually in equilibrium with their respective boronic acids at room temperature. Beside being used in theoretical studies, boroxine is primarily used in the production of optics.
Organovanadium chemistry is the chemistry of organometallic compounds containing a carbon (C) to vanadium (V) chemical bond. Organovanadium compounds find only minor use as reagents in organic synthesis but are significant for polymer chemistry as catalysts.
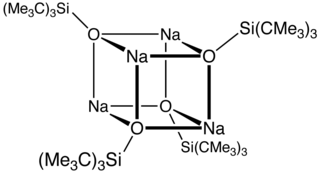
Sodium silox is the name for an organosilicon compound that serves as a source of the siloxide anion [(CH3)3C]3SiO−. Complexes of this bulky anionic ligand often adopt with low coordination numbers. Examples include Ti(silox)3, Nb(silox)3(PMe3), and [Cr(silox)3]−.
Transition metal carbyne complexes are organometallic compounds with a triple bond between carbon and the transition metal. This triple bond consists of a σ-bond and two π-bonds. The HOMO of the carbyne ligand interacts with the LUMO of the metal to create the σ-bond. The two π-bonds are formed when the two HOMO orbitals of the metal back-donate to the LUMO of the carbyne. They are also called metal alkylidynes—the carbon is a carbyne ligand. Such compounds are useful in organic synthesis of alkynes and nitriles. They have been the focus on much fundamental research.
Borane, also known as borine, is an unstable and highly reactive molecule with the chemical formula BH
3. The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. However, the molecular species BH3 is a very strong Lewis acid. Consequently, it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. It normally dimerizes to diborane in the absence of other chemicals.

A carbon nanothread is a sp3-bonded, one-dimensional carbon crystalline nanomaterial. The tetrahedral sp3-bonding of its carbon is similar to that of diamond. Nanothreads are only a few atoms across, more than 300,000 times thinner than a human hair. They consist of a stiff, strong carbon core surrounded by hydrogen atoms. Carbon nanotubes, although also one-dimensional nanomaterials, in contrast have sp2-carbon bonding as is found in graphite. The smallest carbon nanothread has a diameter of only 0.2 nanometers, much smaller than the diameter of a single-wall carbon nanotube.

Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).

In organometallic chemistry, the activation of cyclopropanes by transition metals is a research theme with implications for organic synthesis and homogeneous catalysis. Being highly strained, cyclopropanes are prone to oxidative addition to transition metal complexes. The resulting metallacycles are susceptible to a variety of reactions. These reactions are rare examples of C-C bond activation. The rarity of C-C activation processes has been attributed to Steric effects that protect C-C bonds. Furthermore, the directionality of C-C bonds as compared to C-H bonds makes orbital interaction with transition metals less favorable. Thermodynamically, C-C bond activation is more favored than C-H bond activation as the strength of a typical C-C bond is around 90 kcal per mole while the strength of a typical unactivated C-H bond is around 104 kcal per mole.

Borane carbonyl is the inorganic compound with the formula H3BCO. This colorless gas is the adduct of borane and carbon monoxide. It is usually prepared by combining borane-ether complexes and CO. The compound is mainly of theoretical and pedagogical interest.

A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand.

Arsenic in the solid state can be found as gray, black, or yellow allotropes. These various forms feature diverse structural motifs, with yellow arsenic enabling the widest range of reactivity. In particular, reaction of yellow arsenic with main group and transition metal elements results in compounds with wide-ranging structural motifs, with butterfly, sandwich and realgar-type moieties featuring most prominently.

Hexa(tert-butoxy)ditungsten(III) is a coordination complex of tungsten(III). It is one of the homoleptic alkoxides of tungsten. A red, air-sensitive solid, the complex has attracted academic attention as the precursor to many organotungsten derivatives. It an example of a charge-neutral complex featuring a W≡W bond, arising from the coupling of a pair of d3 metal centers.
Metal-ligand cooperativity (MLC) is a mode of reactivity in which a metal and ligand of a complex are both involved in the bond breaking or bond formation of a substrate during the course of a reaction. This ligand is an actor ligand rather than a spectator, and the reaction is generally only deemed to contain MLC if the actor ligand is doing more than leaving to provide an open coordination site. MLC is also referred to as "metal-ligand bifunctional catalysis." Note that MLC is not to be confused with cooperative binding.
Suzanne Cathleen Bart an American chemist who is a professor of inorganic chemistry at Purdue University. Her group's research focuses on actinide organometallic chemistry, and especially the characterization of low-valent organouranium complexes, actinide complexes with redox-active ligands, and discovery of new reactions that utilize these compounds. Bart's research has applications in the development of carbon-neutral fuel sources and the remediation of polluted sites.
A molecular electron-reservoir complex is one of a class of redox-active systems which can store and transfer electrons stoichiometrically or catalytically without decomposition. The concept of electron-reservoir complexes was introduced by the work of French chemist, Didier Astruc. From Astruc's discoveries, a whole family of thermally stable, neutral, 19-electron iron(I) organometallic complexes were isolated and characterized, and found to have applications in redox catalysis and electrocatalysis. The following page is a reflection of the prototypal electron-reservoir complexes discovered by Didier Astruc.

Trimesitylvanadium (mesityl or Mes = 2,4,6-trimethylphenyl) is one of the organovanadium complexes with vanadium in an oxidation state of 3. This compound was first synthesized by W. Seidel and G. Kreisel in 1974. To prepare this compound, VCl3(THF)3 (THF = tetrahydrofuran) was reacted with Grignard reagent MesMgBr to form a blue solution at room temperature. It is precipitated by the addition of dioxane, which results in a blue solid. It is thermally stable, but it is also an air-sensitive compound.
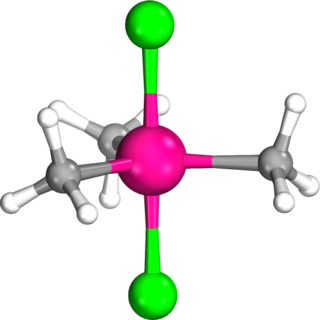
Dichlorotrimethyltantalum, or trimethyltantalum dichloride, is an organotantalum complex with the molecular formula TaCl2(CH3)3. It forms pale yellow, highly air- and water-sensitive crystals which easily sublime in vacuum at room temperature. It was the first reported σ-bonded alkyl complex of tantalum, synthesised by Gordon L. Juvinall at the California Institute of Technology in 1964. It serves as an important precursor for the preparation of a large number of Ta(V) complexes.

Tantalocene trihydride, or bis(η5-cyclopentadienyl)trihydridotantalum, is an organotanalum compound in the family of bent metallocenes consisting of two cyclopentadienyl rings and three hydrides coordinated to a tantalum center. Its formula is TaCp2H3, and it is a white crystalline compound that is sensitive to air. It is the first example of a molecular trihydride of a transition metal.























