
The retina is the innermost, light-sensitive layer of tissue of the eye of most vertebrates and some molluscs. The optics of the eye create a focused two-dimensional image of the visual world on the retina, which then processes that image within the retina and sends nerve impulses along the optic nerve to the visual cortex to create visual perception. The retina serves a function which is in many ways analogous to that of the film or image sensor in a camera.

Retinitis pigmentosa (RP) is a member of a group of genetic disorders called Inherited Retinal Dystrophy (IRD) that cause loss of vision. Symptoms include trouble seeing at night and decreasing peripheral vision. As peripheral vision worsens, people may experience "tunnel vision". Complete blindness is uncommon. Onset of symptoms is generally gradual and often begins in childhood.

A photoreceptor cell is a specialized type of neuroepithelial cell found in the retina that is capable of visual phototransduction. The great biological importance of photoreceptors is that they convert light into signals that can stimulate biological processes. To be more specific, photoreceptor proteins in the cell absorb photons, triggering a change in the cell's membrane potential.

Primary biliary cholangitis (PBC), previously known as primary biliary cirrhosis, is an autoimmune disease of the liver. It results from a slow, progressive destruction of the small bile ducts of the liver, causing bile and other toxins to build up in the liver, a condition called cholestasis. Further slow damage to the liver tissue can lead to scarring, fibrosis, and eventually cirrhosis.

Melanopsin is a type of photopigment belonging to a larger family of light-sensitive retinal proteins called opsins and encoded by the gene Opn4. In the mammalian retina, there are two additional categories of opsins, both involved in the formation of visual images: rhodopsin and photopsin in the rod and cone photoreceptor cells, respectively.

Ursodeoxycholic acid (UDCA), also known as ursodiol, is a secondary bile acid, produced in humans and most other species from metabolism by intestinal bacteria. It is synthesized in the liver in some species, and was first identified in bile of bears of genus Ursus, from which its name derived. In purified form, it has been used to treat or prevent several diseases of the liver or bile ducts.

Lipofuscin is the name given to fine yellow-brown pigment granules composed of lipid-containing residues of lysosomal digestion. It is considered to be one of the aging or "wear-and-tear" pigments, found in the liver, kidney, heart muscle, retina, adrenals, nerve cells, and ganglion cells.
Intrinsically photosensitive retinal ganglion cells (ipRGCs), also called photosensitive retinal ganglion cells (pRGC), or melanopsin-containing retinal ganglion cells (mRGCs), are a type of neuron in the retina of the mammalian eye. The presence of an additional photoreceptor was first suspected in 1927 when mice lacking rods and cones still responded to changing light levels through pupil constriction; this suggested that rods and cones are not the only light-sensitive tissue. However, it was unclear whether this light sensitivity arose from an additional retinal photoreceptor or elsewhere in the body. Recent research has shown that these retinal ganglion cells, unlike other retinal ganglion cells, are intrinsically photosensitive due to the presence of melanopsin, a light-sensitive protein. Therefore, they constitute a third class of photoreceptors, in addition to rod and cone cells.
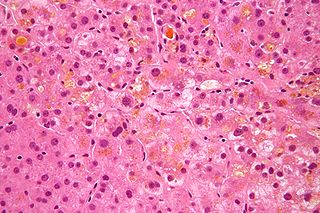
Cholestasis is a condition where the flow of bile from the liver to the duodenum is impaired. The two basic distinctions are:
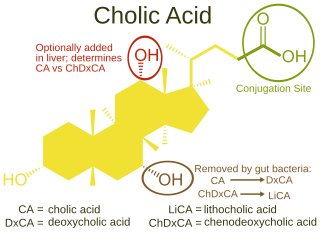
Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Diverse bile acids are synthesized in the liver. Bile acids are conjugated with taurine or glycine residues to give anions called bile salts.

Müller glia, or Müller cells, are a type of retinal glial cells, first recognized and described by Heinrich Müller. They are found in the vertebrate retina, where they serve as support cells for the neurons, as all glial cells do. They are the most common type of glial cell found in the retina. While their cell bodies are located in the inner nuclear layer of the retina, they span across the entire retina.

The photoreceptor cell-specific nuclear receptor (PNR), also known as NR2E3, is a protein that in humans is encoded by the NR2E3 gene. PNR is a member of the nuclear receptor super family of intracellular transcription factors.

Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit beta is the beta subunit of the protein complex PDE6 that is encoded by the PDE6B gene. PDE6 is crucial in transmission and amplification of visual signal. The existence of this beta subunit is essential for normal PDE6 functioning. Mutations in this subunit are responsible for retinal degeneration such as retinitis pigmentosa or congenital stationary night blindness.

Disc shedding is the process by which photoreceptor cells in the retina are renewed. The disc formations in the outer segment of photoreceptors, which contain the photosensitive opsins, are completely renewed every ten days.

Hyodeoxycholic acid, also known as 3α,6α-Dihydroxy-5β-cholan-24-oic acid or HDCA, is a secondary bile acid, one of the metabolic byproducts of intestinal bacteria. It differs from deoxycholic acid in that the 6α-hydroxyl is in the 12 position in the former. The 6α-hydroxyl group makes HDCA a hydrophilic acid, a property it shares with hyocholic acid. HDCA is present in mammalian species in different proportions. It is the main acid constituent of hog bile, and for this reason it was used industrially as precursor for steroid synthesis before total synthesis became practical.
Gene therapy using lentiviral vectors was being explored in early stage trials as of 2009.
Retinal gene therapy holds a promise in treating different forms of non-inherited and inherited blindness.

S-Nitrosoglutathione (GSNO) is an endogenous S-nitrosothiol (SNO) that plays a critical role in nitric oxide (NO) signaling and is a source of bioavailable NO. NO coexists in cells with SNOs that serve as endogenous NO carriers and donors. SNOs spontaneously release NO at different rates and can be powerful terminators of free radical chain propagation reactions, by reacting directly with ROO• radicals, yielding nitro derivatives as end products. NO is generated intracellularly by the nitric oxide synthase (NOS) family of enzymes: nNOS, eNOS and iNOS while the in vivo source of many of the SNOs is unknown. In oxygenated buffers, however, formation of SNOs is due to oxidation of NO to dinitrogen trioxide (N2O3). Some evidence suggests that both exogenous NO and endogenously derived NO from nitric oxide synthases can react with glutathione to form GSNO.
Erythropoietin in neuroprotection is the use of the glycoprotein erythropoietin (Epo) for neuroprotection. Epo controls erythropoiesis, or red blood cell production.
Drug abuse retinopathy is damage to the retina of the eyes caused by chronic drug abuse. Types of retinopathy caused by drug abuse include maculopathy, Saturday night retinopathy, and talc retinopathy. Common symptoms include temporary and permanent vision loss, blurred vision, and night blindness. Substances commonly associated with this condition include poppers, heroin, cocaine, methamphetamine, tobacco, and alcohol.
















