
Azapirones are a class of drugs used as anxiolytics, antidepressants, and antipsychotics. They are commonly used as add-ons to other antidepressants, such as selective serotonin reuptake inhibitors (SSRIs).

Ipsapirone is a selective 5-HT1A receptor partial agonist of the piperazine and azapirone chemical classes. It has antidepressant and anxiolytic effects. Ipsapirone was studied in several placebo-controlled trials for depression and continues to be used in research.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.

Tandospirone, sold under the brand name Sediel, is an anxiolytic and antidepressant medication used in Japan and China, where it is marketed by Dainippon Sumitomo Pharma. It is a member of the azapirone class of drugs and is closely related to other azapirones like buspirone and gepirone.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

8-OH-DPAT is a research chemical of the aminotetralin chemical class which was developed in the 1980s and has been widely used to study the function of the 5-HT1A receptor. It was one of the first major 5-HT1A receptor full agonists to have been discovered.

WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor. It is sometimes referred to as a silent antagonist at the former receptor. It is closely related to WAY-100135.

Binospirone (MDL-73,005-EF) is a drug which acts as a partial agonist at 5-HT1A somatodendritic autoreceptors but as an antagonist at postsynaptic 5-HT1A receptors. It has anxiolytic effects.

Perospirone (Lullan) is an atypical antipsychotic of the azapirone family. It was introduced in Japan by Dainippon Sumitomo Pharma in 2001 for the treatment of schizophrenia and acute cases of bipolar mania.

Zalospirone (WY-47,846) is a selective 5-HT1A partial agonist of the azapirone chemical class. It was found to be effective in the treatment of anxiety and depression in clinical trials, but a high proportion of subjects dropped out due to side effects and development was subsequently never completed.

Alnespirone (S-20,499) is a selective 5-HT1A receptor full agonist of the azapirone chemical class. It has antidepressant and anxiolytic effects.
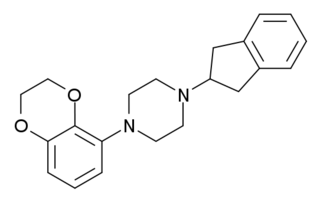
S-15535 is a phenylpiperazine drug which is a potent and highly selective 5-HT1A receptor ligand that acts as an agonist and antagonist at the presynaptic and postsynaptic 5-HT1A receptors, respectively. It has anxiolytic properties.

Oxaflozane (INN) (brand name Conflictan) is an antidepressant and anxiolytic drug that was introduced by Solvay in France in 1982 for the treatment of depression but has since been discontinued. It is a prodrug of flumexadol (N-dealkyloxaflozane; 2-(3-trifluoromethylphenyl)morpholine; CERM-1841 or 1841-CERM), which is reported to act as an agonist of the serotonin 5-HT1A (pKi = 7.1) and 5-HT2C (pKi = 7.5) receptors and, to a much lesser extent, of the 5-HT2A (pKi = 6.0) receptor. In addition to its serotonergic properties, oxaflozane may also produce anticholinergic side effects at high doses, namely in overdose.

Eptapirone (F-11,440) is a very potent and highly selective 5-HT1A receptor full agonist of the azapirone family. Its affinity for the 5-HT1A receptor was reported to be 4.8 nM (Ki), and its intrinsic activity approximately equal to that of serotonin.
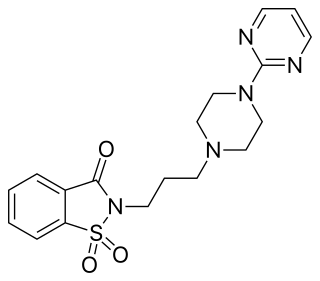
Revospirone is an azapirone drug which was patented as a veterinary tranquilizer but was never marketed. It acts as a selective 5-HT1A receptor partial agonist. Similarly to other azapirones such as buspirone, revospirone produces 1-(2-pyrimidinyl)piperazine (1-PP) as an active metabolite. As a result, it also acts as an α2-adrenergic receptor antagonist to an extent.

Umespirone (KC-9172) is a drug of the azapirone class which possesses anxiolytic and antipsychotic properties. It behaves as a 5-HT1A receptor partial agonist (Ki = 15 nM), D2 receptor partial agonist (Ki = 23 nM), and α1-adrenoceptor receptor antagonist (Ki = 14 nM), and also has weak affinity for the sigma receptor (Ki = 558 nM). Unlike many other anxiolytics and antipsychotics, umespirone produces minimal sedation, cognitive/memory impairment, catalepsy, and extrapyramidal symptoms.

Lesopitron (E-4424) is a selective full agonist of the 5-HT1A receptor which is structurally related to the azapirones. In 2001 it was under development by Esteve as an anxiolytic for the treatment of generalized anxiety disorder (GAD). It made it to phase II clinical trials but was apparently discontinued as no new information on lesopitron has surfaced since.

Osemozotan (MKC-242) is a selective 5-HT1A receptor agonist with some functional selectivity, acting as a full agonist at presynaptic and a partial agonist at postsynaptic 5-HT1A receptors. 5-HT1A receptor stimulation influences the release of various neurotransmitters including serotonin, dopamine, norepinephrine, and acetylcholine. 5-HT1A receptors are inhibitory G protein-coupled receptor.

F-15,063 is an orally active potential antipsychotic, and an antagonist at the D2/D3 receptors, partial agonist at the D4 receptor, and agonist at the 5-HT1A receptors. It has greater efficacy at the 5-HT1A receptors than other antipsychotics, such as clozapine, aripiprazole, and ziprasidone. This greater efficacy may lead to enhanced antipsychotic properties, as antipsychotics that lack 5-HT1A affinity are associated with increased risk of extrapyramidal symptoms, and lack of activity against the negative symptoms of schizophrenia.
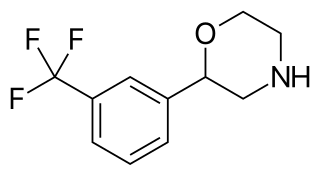
Flumexadol (INN) is a drug described and researched as a non-opioid analgesic which was never marketed. It has been found to act as an agonist of the serotonin 5-HT1A and 5-HT2C receptors and, to a much lesser extent, of the 5-HT2A receptor. According to Nilsson (2006) in a paper on 5-HT2C receptor agonists as potential anorectics, "The (+)-enantiomer of this compound showed [...] affinity for the 5-HT2C receptor (Ki) 25 nM) [...] and was 40-fold selective over the 5-HT2A receptor in receptor binding studies. Curiously, the racemic version [...], also known as 1841 CERM, was originally reported to possess analgesic properties while no association with 5-HT2C receptor activity was mentioned." It is implied that flumexadol might be employable as an anorectic in addition to analgesic. Though flumexadol itself has never been approved for medical use, oxaflozane is a prodrug of the compound that was formerly used clinically in France as an antidepressant and anxiolytic agent.




















