
Zydus Lifesciences Limited, formerly known as Cadila Healthcare Limited, is an Indian multinational pharmaceutical company headquartered in Ahmedabad, which is primarily engaged in the manufacturing of generic drugs. The company ranked 100th in the Fortune India 500 list in 2020.
Inovio Pharmaceuticals is an American biotechnology company focused on the discovery, development, and commercialization of synthetic DNA products for treating cancers and infectious diseases. In April 2020, Inovio was among some 100 companies, academic centers, or research organizations developing a vaccine candidate for treating people infected with COVID-19, with more than 170 total vaccine candidates in development.
A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID‑19).
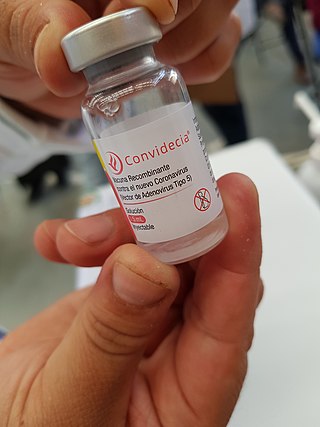
AD5-nCOV, trade-named Convidecia, is a single-dose viral vector vaccine for COVID-19 that is also used as an inhaled booster. It was developed by CanSino Biologics, with Phase III trials conducted in Argentina, Chile, Mexico, Pakistan, Russia, and Saudi Arabia with 40,000 participants.

Covaxin is a whole inactivated virus-based COVID-19 vaccine developed by Bharat Biotech in collaboration with the Indian Council of Medical Research - National Institute of Virology.

India began administration of COVID-19 vaccines on 16 January 2021. As of 4 March 2023, India has administered over 2.2 billion doses overall, including first, second and precautionary (booster) doses of the currently approved vaccines. In India, 95% of the eligible population (12+) has received at least one shot, and 88% of the eligible population (12+) is fully vaccinated.
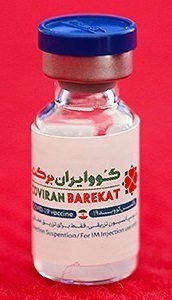
COVIran Barekat is a COVID-19 vaccine developed in Iran by Shifa Pharmed Industrial Group, a subsidiary of the Barkat Pharmaceutical Group. It is an inactivated virus-based vaccine. Iranian authorities have authorized its emergency use. This makes it the first locally developed COVID-19 vaccine to be approved for emergency use in the Middle East.

The CureVac COVID-19 vaccine was a COVID-19 vaccine candidate developed by CureVac N.V. and the Coalition for Epidemic Preparedness Innovations (CEPI). The vaccine showed inadequate results in its Phase III trials with only 47% efficacy. In October 2021 CureVac abandoned further development and production plans for CVnCoV and refocused efforts on a cooperation with GlaxoSmithKline.

Inovio COVID-19 vaccine is a COVID-19 vaccine candidate developed by Inovio Pharmaceuticals.

SCB-2019 is a protein subunit COVID-19 vaccine developed by Clover Biopharmaceuticals using an adjuvant from Dynavax technologies. Positive results of Phase I trials for the vaccine were published in The Lancet and the vaccine completed enrollment of 29,000 participants in Phase II/III trials in July 2021. In September 2021, SCB-2019 announced Phase III results showing 67% efficacy against all cases of COVID-19 and 79% efficacy against all cases of the Delta variant. Additionally, the vaccine was 84% effective against moderate cases and 100% effective against hospitalization.

The Sanofi–GSK COVID-19 vaccine sold under the brand name VidPrevtyn Beta, is a COVID-19 vaccine developed by Sanofi Pasteur and GSK.

BriLife, also known as IIBR-100, is a replication-competent recombinant VSV viral vectored COVID-19 vaccine candidate. It was developed by the Israel Institute for Biological Research (IIBR). The IIBR partnered with the US-based NRx Pharmaceuticals to complete clinical trials and commercialize the vaccine. A study conducted in hamsters suggested that one dose of the vaccine was safe and effective at protecting against COVID-19.

The Sinopharm WIBP COVID-19 vaccine, also known as WIBP-CorV, is one of two inactivated virus COVID-19 vaccines developed by Sinopharm. Peer-reviewed results show that the vaccine is 72.8% effective against symptomatic cases and 100% against severe cases. The other inactivated virus COVID-19 vaccine developed by Sinopharm is the BIBP vaccine (BBIBP-CorV) which is comparably more successful. 1 billion doses are expected to be produced per year.

Corbevax is a protein subunit COVID-19 vaccine developed by Texas Children's Hospital Center for Vaccine Development and Baylor College of Medicine in Houston, Texas and Dynavax technologies based in Emeryville, California. It is licensed to Indian biopharmaceutical firm Biological E. Limited (BioE) for development and production.

HGC019 is a mRNA and Self-amplifying mRNA (saRNA) based COVID-19 vaccine candidate being developed by Gennova Biopharmaceuticals and HDT Bio Corp. with active support from NIH under The Indo-US Vaccine Action Program (VAP) and Department of Biotechnology, India.

V-01 is a protein subunit COVID-19 vaccine candidate developed by a subsidiary of Livzon Pharmaceutical Group Inc.
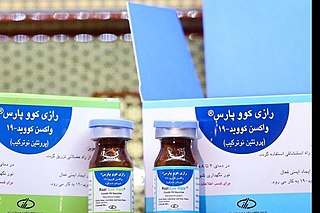
Razi Cov Pars is a COVID-19 vaccine developed by the Iranian Razi Vaccine and Serum Research Institute Razi Cov Pars is a covid-19 vaccine based on recombinant protein, which is being produced by Razi Vaccine and Serum Research Institute, Iran. This vaccine is the first injectable-intranasal recombinant protein corona vaccine.It's the second Iranian COVID-19 vaccine reaching human trials and is currently in phase III of clinical research during which it's compared to the Sinopharm BIBP vaccine.

COVAX-19 is a recombinant protein-based COVID-19 vaccine developed by South Australian-based biotech company Vaxine, in collaboration with CinnaGen, a private company with operations in the Middle East. It is under clinical trial in collaboration with the Iranian company CinnaGen.

COVID-19 vaccine clinical research uses clinical research to establish the characteristics of COVID-19 vaccines. These characteristics include efficacy, effectiveness, and safety. As of November 2022, 40 vaccines are authorized by at least one national regulatory authority for public use:

S-268019-b is a protein subunit COVID-19 vaccine candidate developed by Shionogi.









