
Asthma is a long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms. Symptoms include episodes of wheezing, coughing, chest tightness, and shortness of breath. These may occur a few times a day or a few times per week. Depending on the person, asthma symptoms may become worse at night or with exercise.

Anaphylaxis is a serious, potentially fatal allergic reaction and medical emergency that is rapid in onset and requires immediate medical attention regardless of use of emergency medication on site. It typically causes more than one of the following: an itchy rash, throat closing due to swelling that can obstruct or stop breathing; severe tongue swelling that can also interfere with or stop breathing; shortness of breath, vomiting, lightheadedness, loss of consciousness, low blood pressure, and medical shock. These symptoms typically start in minutes to hours and then increase very rapidly to life-threatening levels. Urgent medical treatment is required to prevent serious harm and death, even if the patient has used an epipen or has taken other medications in response, and even if symptoms appear to be improving.

Rhinitis, also known as coryza, is irritation and inflammation of the mucous membrane inside the nose. Common symptoms are a stuffy nose, runny nose, sneezing, and post-nasal drip.
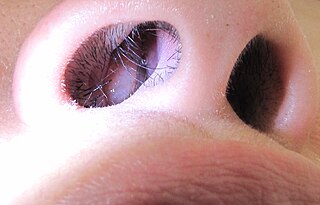
Nasal polyps (NP) are noncancerous growths within the nose or sinuses. Symptoms include trouble breathing through the nose, loss of smell, decreased taste, post nasal drip, and a runny nose. The growths are sac-like, movable, and nontender, though face pain may occasionally occur. They typically occur in both nostrils in those who are affected. Complications may include sinusitis and broadening of the nose.

Leukotrienes are a family of eicosanoid inflammatory mediators produced in leukocytes by the oxidation of arachidonic acid (AA) and the essential fatty acid eicosapentaenoic acid (EPA) by the enzyme arachidonate 5-lipoxygenase.

Allergen immunotherapy, also known as desensitization or hypo-sensitization, is a medical treatment for environmental allergies, such as insect bites, and asthma. Immunotherapy involves exposing people to larger and larger amounts of allergens in an attempt to change the immune system's response.
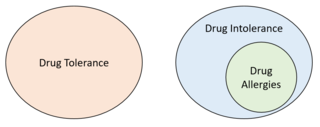
Drug intolerance or drug sensitivity refers to an inability to tolerate the adverse effects of a medication, generally at therapeutic or subtherapeutic doses. Conversely, a patient is said to be "tolerating" a drug when they can tolerate its adverse effects. Some instances of drug intolerance are known to result from genetic variations in drug metabolism.

Salicylate sensitivity is any adverse effect that occurs when a usual amount of salicylate is ingested. People with salicylate intolerance are unable to consume a normal amount of salicylate without adverse effects.

Leukotriene E4 (LTE4) is a cysteinyl leukotriene involved in inflammation. It is known to be produced by several types of white blood cells, including eosinophils, mast cells, tissue macrophages, and basophils, and recently was also found to be produced by platelets adhering to neutrophils. It is formed from the sequential conversion of LTC4 to LTD4 and then to LTE4, which is the final and most stable cysteinyl leukotriene. Compared to the short half lives of LTC4 and LTD4, LTE4 is relatively stable and accumulates in breath condensation, in plasma, and in urine, making it the dominant cysteinyl leukotriene detected in biologic fluids. Therefore, measurements of LTE4, especially in the urine, are commonly monitored in clinical research studies.
Arachidonate 5-lipoxygenase, also known as ALOX5, 5-lipoxygenase, 5-LOX, or 5-LO, is a non-heme iron-containing enzyme that in humans is encoded by the ALOX5 gene. Arachidonate 5-lipoxygenase is a member of the lipoxygenase family of enzymes. It transforms essential fatty acids (EFA) substrates into leukotrienes as well as a wide range of other biologically active products. ALOX5 is a current target for pharmaceutical intervention in a number of diseases.

Cysteinyl leukotriene receptor 1, also termed CYSLTR1, is a receptor for cysteinyl leukotrienes (LT). CYSLTR1, by binding these cysteinyl LTs contributes to mediating various allergic and hypersensitivity reactions in humans as well as models of the reactions in other animals.
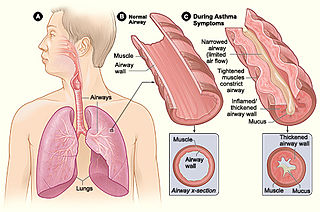
Asthma is a common pulmonary condition defined by chronic inflammation of respiratory tubes, tightening of respiratory smooth muscle, and episodes of bronchoconstriction. The Centers for Disease Control and Prevention estimate that 1 in 11 children and 1 in 12 adults have asthma in the United States of America. According to the World Health Organization, asthma affects 235 million people worldwide. There are two major categories of asthma: allergic and non-allergic. The focus of this article will be allergic asthma. In both cases, bronchoconstriction is prominent.
Max Samter was a German-American immunologist who first extensively studied the triad between asthma, aspirin allergy, and nasal polyps that became known as Samter's triad, now aspirin-exacerbated respiratory disease. Samter was a third generation doctor and obtained medical training in Europe. After fleeing Nazi occupation in Germany, Samter had a long career in medical research in the United States. He is a pioneer in the field of immunology, having written many of the foundational textbooks of the field. Samter founded The Max Samter Institute for Immunology Research at Grant Hospital in Chicago, and after his death it was renamed in his honor.

Dupilumab, sold under the brand name Dupixent, is a monoclonal antibody blocking interleukin 4 and interleukin 13, used for allergic diseases such as atopic dermatitis (eczema), asthma and nasal polyps which result in chronic sinusitis. It is also used for the treatment of eosinophilic esophagitis and prurigo nodularis.
Mast cell activation syndrome (MCAS) is a term referring to one of two types of mast cell activation disorder (MCAD); the other type is idiopathic MCAD. MCAS is an immunological condition in which mast cells inappropriately and excessively release chemical mediators, resulting in a range of chronic symptoms, sometimes including anaphylaxis or near-anaphylaxis attacks. Primary symptoms include cardiovascular, dermatological, gastrointestinal, neurological and respiratory problems.
Alcohol-induced respiratory reactions, also termed alcohol-induced asthma and alcohol-induced respiratory symptoms, are increasingly recognized as a pathological bronchoconstriction response to the consumption of alcohol that afflicts many people with a "classical" form of asthma, the airway constriction disease evoked by the inhalation of allergens. Alcohol-induced respiratory reactions reflect the operation of different and often racially related mechanisms that differ from those of classical, allergen-induced asthma.
NSAIDhypersensitivity reactions encompass a broad range of allergic or allergic-like symptoms that occur within minutes to hours after ingesting aspirin or other NSAID nonsteroidal anti-inflammatory drugs. Hypersensitivity drug reactions differ from drug toxicity reactions in that drug toxicity reactions result from the pharmacological action of a drug, are dose-related, and can occur in any treated individual. Hypersensitivity reactions are idiosyncratic reactions to a drug. Although the term NSAID was introduced to signal a comparatively low risk of adverse effects, NSAIDs do evoke a broad range of hypersensitivity syndromes. These syndromes have recently been classified by the European Academy of Allergy and Clinical Immunology Task Force on NSAIDs Hypersensitivity.

Asthma triggers are factors or stimuli that provoke the exacerbation of asthma symptoms or increase the degree of airflow disruption, which can lead to an asthma attack. An asthma attack is characterized by an obstruction of the airway, hypersecretion of mucus and bronchoconstriction due to the contraction of smooth muscles around the respiratory tract. Its symptoms include a wide range of manifestations such as breathlessness, coughing, a tight chest and wheezing.

Lysine acetylsalicylate, also known as aspirin DL-lysine or lysine aspirin, is a more soluble form of acetylsalicylic acid (aspirin). As with aspirin itself, it is a nonsteroidal anti-inflammatory drug (NSAID) with analgesic, anti-inflammatory, antithrombotic and antipyretic properties. It is composed of the ammonium form of the amino acid lysine paired with the conjugate base of aspirin.
Asthma phenotyping and endotyping is a novel approach to asthma classification inspired by precision medicine. It seeks to separate the clinical presentations or clusters of signs and symptoms of asthma, known as asthma phenotypes, from their underlying etiologies or causes, known as asthma endotypes.

















