Sinopharm Group Co., Ltd. is a Chinese pharmaceutical company. The parent company of Sinopharm Group was Sinopharm Industrial Investment, a 51–49 joint venture of state-owned enterprise China National Pharmaceutical Group and civilian-run enterprise Fosun Pharmaceutical.

China National Pharmaceutical Group Corporation (CNPGC), commonly referred to as Sinopharm, is a Chinese state-owned enterprise. The corporation was the indirect major shareholder of publicly traded companies Sinopharm Group, China Traditional Chinese Medicine, Shanghai Shyndec Pharmaceutical, and Beijing Tiantan Biological Products.
The COVID-19 pandemic in Hungary was a part of the worldwide pandemic of coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2. On 4 March 2020, the first cases in Hungary were announced. The first coronavirus-related death was announced on 15 March on the government's official website.

A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID‑19).

BioNTech SE is a German biotechnology company based in Mainz that develops and manufactures active immunotherapies for patient-specific approaches to the treatment of diseases. It develops pharmaceutical candidates based on messenger ribonucleic acid (mRNA) for use as individualized cancer immunotherapies, as vaccines against infectious diseases and as protein replacement therapies for rare diseases, and also engineered cell therapy, novel antibodies and small molecule immunomodulators as treatment options for cancer.
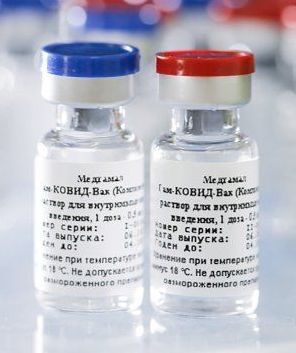
Sputnik V or Gam-COVID-Vac is an adenovirus viral vector vaccine for COVID-19 developed by the Gamaleya Research Institute of Epidemiology and Microbiology in Russia. It is the world's first registered combination vector vaccine for the prevention of COVID-19, having been registered on 11 August 2020 by the Russian Ministry of Health.

Vaccine diplomacy, a form of medical diplomacy, is the use of vaccines to improve a country's diplomatic relationship and influence of other countries. Meanwhile, vaccine diplomacy also "means a set of diplomatic measures taken to ensure access to the best practices in the development of potential vaccines, to enhance bilateral and/or multilateral cooperation between countries in conducting joint R&D, and, in the case of the announcement of production, to ensure the signing of a contract for the purchase of the vaccine at the shortest term." Although primary discussed in the context of the supply of COVID-19 vaccines, it also played a part in the distribution of the smallpox vaccine.

The Sinopharm BIBP COVID-19 vaccine, also known as BBIBP-CorV, the Sinopharm COVID-19 vaccine, or BIBP vaccine, is one of two whole inactivated virus COVID-19 vaccines developed by Sinopharm's Beijing Institute of Biological Products. It completed Phase III trials in Argentina, Bahrain, Egypt, Morocco, Pakistan, Peru, and the United Arab Emirates (UAE) with over 60,000 participants. BBIBP-CorV shares similar technology with CoronaVac and Covaxin, other inactivated virus vaccines for COVID-19. Its product name is SARS-CoV-2 Vaccine, not to be confused with the similar product name of CoronaVac.
The COVID-19 vaccination program in the Philippines was a mass immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the pandemic in the country. The vaccination program was initiated by the Duterte administration on March 1, 2021, a day after the arrival of the country's first vaccine doses which were donated by the Chinese government.

The COVID-19 vaccination campaign in Russia is an ongoing mass immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country. Russia became the first country to begin a mass COVID-19 vaccination programme on 5 December 2020, starting with primarily doctors, medical workers and teachers. In January 2021, this was extended to the entire population.

On 22 February 2021, Zimbabwe launched their national COVID-19 vaccination program using the Sinopharm BIBP vaccine. As of 17 June 2022, 6,260,228 people have received their first dose, 4,598,703 have received their second dose, and 851,874 have received a third dose.
The COVID-19 vaccination in Morocco is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.

Sputnik Light is a single dose COVID-19 vaccine developed by the Gamaleya Research Institute of Epidemiology and Microbiology. It consists of the first dose of the Sputnik V vaccine, which is based on the Ad26 vector, and it can be stored at a normal refrigerator temperature of 2–8 °C (36–46 °F). The institute says this version would be ideally suited for areas with acute outbreaks, allowing more people to be vaccinated quickly. It will also be used as a third (booster) dose for those who received Sputnik V at least 6 months earlier.

COVID-19 vaccination in Sri Lanka is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, in response to the ongoing pandemic in the country. As of late July, the Sinopharm BIBP vaccine accounted for 78% of the total 13.8 million vaccines obtained by Sri Lanka to date. The United States donated over 1.5 million Moderna vaccine through COVAX.
COVID-19 vaccination in Burundi is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country. Burundi was one of the last nation states in the world to commence vaccination against COVID-19. This was mostly due to the government's refusal to vaccinate the population throughout most of 2021. In February 2021, Thaddee Ndikumana, the health minister of Burundi, said his country was more concerned with prevention measures. "Since more than 95% of patients are recovering, we estimate that the vaccines are not yet necessary," local media reported.
COVID-19 vaccination in Equatorial Guinea is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.
COVID-19 vaccination in Comoros is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.
The COVID-19 vaccination in the United Arab Emirates is an ongoing mass immunization campaign, in response to the ongoing pandemic.

COVID-19 vaccine clinical research uses clinical research to establish the characteristics of COVID-19 vaccines. These characteristics include efficacy, effectiveness, and safety. As of November 2022, 40 vaccines are authorized by at least one national regulatory authority for public use:









