
COVID-19 Vaccines Global Access, abbreviated as COVAX, is a worldwide initiative aimed at equitable access to COVID-19 vaccines directed by the GAVI vaccine alliance, the Coalition for Epidemic Preparedness Innovations (CEPI), and the World Health Organization (WHO), alongside key delivery partner UNICEF. It is one of the four pillars of the Access to COVID-19 Tools Accelerator, an initiative begun in April 2020 by the WHO, the European Commission, and the government of France as a response to the COVID-19 pandemic. COVAX coordinates international resources to enable low-to-middle-income countries equitable access to COVID-19 tests, therapies, and vaccines. UNICEF is the key delivery partner, leveraging its experience as the largest single vaccine buyer in the world and working on the procurement of COVID-19 vaccine doses, as well as logistics, country readiness and in-country delivery.

The Sinopharm BIBP COVID-19 vaccine, also known as BBIBP-CorV, the Sinopharm COVID-19 vaccine, or BIBP vaccine, is one of two whole inactivated virus COVID-19 vaccines developed by Sinopharm's Beijing Institute of Biological Products. It completed Phase III trials in Argentina, Bahrain, Egypt, Morocco, Pakistan, Peru, and the United Arab Emirates (UAE) with over 60,000 participants. BBIBP-CorV shares similar technology with CoronaVac and Covaxin, other inactivated virus vaccines for COVID-19. Its product name is SARS-CoV-2 Vaccine, not to be confused with the similar product name of CoronaVac.
The COVID-19 vaccination program in the Philippines was a mass immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the pandemic in the country. The vaccination program was initiated by the Duterte administration on March 1, 2021, a day after the arrival of the country's first vaccine doses which were donated by the Chinese government.

The COVID-19 vaccination campaign in Italy is a mass immunization campaign that was put in place by the Italian government in order to respond to the ongoing COVID-19 pandemic. It started on 27 December 2020, together with most countries in the European Union.

The general COVID-19 vaccination in Australia program began on 22 February 2021 in response to the COVID-19 pandemic, with the goal of vaccinating all willing people in Australia before 2022. Front-line workers and aged care staff and residents had priority for being inoculated, before a gradual phased release to less-vulnerable and lower-risk population groups throughout 2021. The Therapeutic Goods Administration (TGA) approved four vaccines for Australian use in 2021: the Pfizer–BioNTech vaccine on 25 January, the Oxford–AstraZeneca vaccine on 16 February, Janssen vaccine on 25 June and the Moderna vaccine on 9 August. Although approved for use, the Janssen vaccine was not included in the Australian vaccination program as of June 2021.

The COVID-19 vaccination campaign in Albania is a mass immunization campaign that was put in place by the Albanian authorities in order to respond to the ongoing COVID-19 pandemic. It started on 11 January 2021.
The COVID-19 vaccination in Morocco is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.
COVID-19 vaccination in Angola is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country. As of 15 June 2021, Angola has administered 1,314,375 doses of vaccines.822,109 people with the first dose and 492,266 people fully vaccinated. Angola began their vaccination program shortly after receiving their first shipment of Oxford AstraZeneca vaccine in early March 2021.
COVID-19 vaccination in Botswana is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.
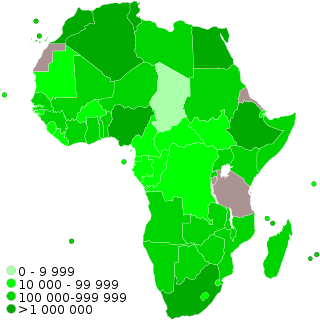
COVID-19 vaccination programs are ongoing in the majority countries and territories in Africa, with 51 of 54 African countries having launched vaccination programs by July 2021. As of October 2023, 51.8% of the continent's population is fully vaccinated with over 1084.5 million doses administered.

Bhutan has promised a free COVID-19 vaccination to all of its citizens, both inside and outside the country. It started mass vaccinations on 27 March 2021.

The COVID-19 vaccination in Vietnam is an ongoing immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in response to the ongoing pandemic in the country. Following the approval of the Oxford–AstraZeneca COVID-19 vaccine on 30 January 2021, vaccinations commenced on 8 March 2021, and will continue throughout the year with the goal of vaccinating 80% of the population by June 2022. The Sputnik V was later approved for use on 23 March 2021. The Sinopharm BIBP vaccine was approved for emergency use on 4 June 2021, while Pfizer–BioNTech COVID-19 vaccine, Moderna COVID-19 vaccine and Janssen COVID-19 vaccine were approved on 12 June 2021, 29 June 2021, and 15 July 2021, respectively. Vietnam approved Abdala vaccine from Center for Genetic Engineering and Biotechnology on 18 September 2021, and Covaxin from Bharat Biotech on 10 November 2021.

COVID-19 vaccination in Mexico is an ongoing immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.

The COVID-19 vaccination in Indonesia is an ongoing mass immunization in response to the COVID-19 pandemic in Indonesia. On 13 January 2021, the program commenced when President Joko Widodo was vaccinated at the presidential palace. In terms of total doses given, Indonesia ranks third in Asia and fifth in the world.

Bangladesh began the administration of COVID-19 vaccines on 27 January 2021 while mass vaccination started on 7 February 2021.

COVID-19 vaccination in Sri Lanka is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, in response to the ongoing pandemic in the country. As of late July, the Sinopharm BIBP vaccine accounted for 78% of the total 13.8 million vaccines obtained by Sri Lanka to date. The United States donated over 1.5 million Moderna vaccine through COVAX.

COVID-19 vaccination in Taiwan is an ongoing immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in response to the ongoing pandemic in the country.
COVID-19 vaccination in Egypt is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.













