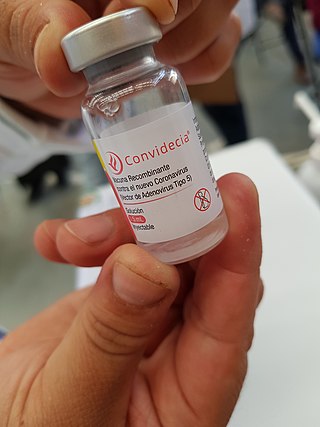
AD5-nCOV, trade-named Convidecia, is a single-dose viral vector vaccine for COVID-19 that is also used as an inhaled booster. It was developed by CanSino Biologics, with Phase III trials conducted in Argentina, Chile, Mexico, Pakistan, Russia, and Saudi Arabia with 40,000 participants.

Valneva COVID-19 vaccine is a COVID-19 vaccine developed by French biotechnology company Valneva SE in collaboration with the American biopharmaceutical company Dynavax Technologies.

ZF2001, trade-named Zifivax or ZF-UZ-VAC-2001, is an adjuvanted protein subunit COVID-19 vaccine developed by Anhui Zhifei Longcom in collaboration with the Institute of Microbiology at the Chinese Academy of Sciences. The vaccine candidate is in Phase III trials with 29,000 participants in China, Ecuador, Malaysia, Pakistan, and Uzbekistan.
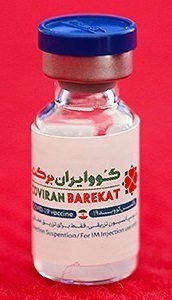
COVIran Barekat is a COVID-19 vaccine developed in Iran by Shifa Pharmed Industrial Group, a subsidiary of the Barkat Pharmaceutical Group. It is an inactivated virus-based vaccine. Iranian authorities have authorized its emergency use. This makes it the first locally developed COVID-19 vaccine to be approved for emergency use in the Middle East.

The CureVac COVID-19 vaccine was a COVID-19 vaccine candidate developed by CureVac N.V. and the Coalition for Epidemic Preparedness Innovations (CEPI). The vaccine showed inadequate results in its Phase III trials with only 47% efficacy. In October 2021 CureVac abandoned further development and production plans for CVnCoV and refocused efforts on a cooperation with GlaxoSmithKline.

ZyCoV-D is a DNA plasmid-based COVID-19 vaccine developed by Indian pharmaceutical company Cadila Healthcare, with support from the Biotechnology Industry Research Assistance Council. It is approved for emergency use in India.

The Sanofi–GSK COVID-19 vaccine sold under the brand name VidPrevtyn Beta, is a COVID-19 vaccine developed by Sanofi Pasteur and GSK.

iNCOVACC is an intranasal COVID-19 vaccine candidate developed by Bharat Biotech, American company Precision Virologics and the Washington University School of Medicine in St Louis, Missouri, United States.

The Sinopharm WIBP COVID-19 vaccine, also known as WIBP-CorV, is one of two inactivated virus COVID-19 vaccines developed by Sinopharm. Peer-reviewed results show that the vaccine is 72.8% effective against symptomatic cases and 100% against severe cases. The other inactivated virus COVID-19 vaccine developed by Sinopharm is the BIBP vaccine (BBIBP-CorV) which is comparably more successful. 1 billion doses are expected to be produced per year.

Minhai COVID-19 vaccine, trademarked as KCONVAC, is a COVID-19 vaccine developed by Shenzhen Kangtai Biological Products Co. Ltd and its subsidiary, Beijing Minhai Biotechnology Co., Ltd.

Corbevax is a protein subunit COVID-19 vaccine developed by Texas Children's Hospital Center for Vaccine Development and Baylor College of Medicine in Houston, Texas and Dynavax technologies based in Emeryville, California. It is licensed to Indian biopharmaceutical firm Biological E. Limited (BioE) for development and production.

HGC019 is a mRNA based COVID-19 vaccine candidate being developed by Gennova Biopharmaceuticals and HDT Bio Corp. with active support from NIH under The Indo-US Vaccine Action Program (VAP) and Department of Biotechnology, India.
EuCorVac-19 is a COVID-19 vaccine candidate developed by EuBiologics Co.

V-01 is a protein subunit COVID-19 vaccine candidate developed by a subsidiary of Livzon Pharmaceutical Group Inc.
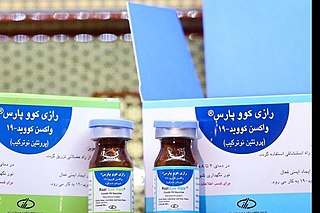
Razi Cov Pars is a COVID-19 vaccine developed by the Iranian Razi Vaccine and Serum Research Institute.

mRNA-1283 is a COVID-19 vaccine candidate developed by Moderna.

ARCT-154, also known as VBC-COV19-154 in Vietnam, is a COVID-19 vaccine candidate developed by Arcturus Therapeutics. For its development, Arcturus collaborated with Vinbiocare, a Vietnamese company, for support with clinical trials and manufacturing.

AdCLD-CoV19 is a COVID-19 vaccine candidate developed by Cellid Co, a company from South Korea.

COVID-19 vaccine clinical research uses clinical research to establish the characteristics of COVID-19 vaccines. These characteristics include efficacy, effectiveness and safety. As of November 2022, 40 vaccines are authorized by at least one national regulatory authority for public use:

LYB001 is a COVID-19 vaccine candidate developed by Yantai Patronus Biotech Co., Ltd.




