The Therapeutic Goods Administration (TGA) is the medicine and therapeutic regulatory agency of the Australian Government. As part of the Department of Health and Aged Care, the TGA regulates the quality, supply and advertising of medicines, pathology devices, medical devices, blood products and most other therapeutics. Any items that claim to have a therapeutic effect, are involved in the administration of medication, or are otherwise covered by the Therapeutic Goods Act 1989, the Therapeutic Goods Regulations 1990, or a ministerial order, must be approved by the TGA and registered in the Australian Register of Therapeutic Goods.
Serum Institute of India (SII) is an Indian biotechnology and biopharmaceuticals company, based in Pune. It was founded by Cyrus Poonawalla in 1966 and is a part of Cyrus Poonawalla Group.

The Oxford–AstraZeneca COVID‑19 vaccine, sold under the brand names Covishield and Vaxzevria among others, is a viral vector vaccine for the prevention of COVID-19. It was developed in the United Kingdom by Oxford University and British-Swedish company AstraZeneca, using as a vector the modified chimpanzee adenovirus ChAdOx1. The vaccine is given by intramuscular injection. Studies carried out in 2020 showed that the efficacy of the vaccine is 76.0% at preventing symptomatic COVID-19 beginning at 22 days following the first dose and 81.3% after the second dose. A study in Scotland found that, for symptomatic COVID-19 infection after the second dose, the vaccine is 81% effective against the Alpha variant and 61% against the Delta variant.
The COVID-19 vaccination programme in the United Kingdom is an ongoing mass immunisation campaign for coronavirus disease 2019 (COVID-19) during the COVID-19 pandemic in the United Kingdom.
The COVID-19 vaccination program in the Philippines was a mass immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the pandemic in the country. The vaccination program was initiated by the Duterte administration on March 1, 2021, a day after the arrival of the country's first vaccine doses which were donated by the Chinese government.

The general COVID-19 vaccination in Australia program began on 22 February 2021 in response to the COVID-19 pandemic, with the goal of vaccinating all willing people in Australia before 2022. Front-line workers and aged care staff and residents had priority for being inoculated, before a gradual phased release to less-vulnerable and lower-risk population groups throughout 2021. The Therapeutic Goods Administration (TGA) approved four vaccines for Australian use in 2021: the Pfizer–BioNTech vaccine on 25 January, the Oxford–AstraZeneca vaccine on 16 February, Janssen vaccine on 25 June and the Moderna vaccine on 9 August. Although approved for use, the Janssen vaccine was not included in the Australian vaccination program as of June 2021.

The COVID-19 vaccination campaign in Albania is a mass immunization campaign that was put in place by the Albanian authorities in order to respond to the ongoing COVID-19 pandemic. It started on 11 January 2021.

COVID-19 vaccination in Canada is an ongoing, intergovernmental effort coordinated between the bodies responsible in the Government of Canada to acquire and distribute vaccines to individual provincial and territorial governments who in turn administer authorized COVID-19 vaccines during the COVID-19 pandemic in Canada. Provinces have worked with local municipal governments, hospital systems, family doctors and independently owned pharmacies to aid in part, or in full with vaccination rollout. The vaccination effort in full is the largest such immunization effort in the nation's history. The vaccination effort began December 14, 2020, and is currently ongoing.

COVID-19 vaccination in South Africa is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.
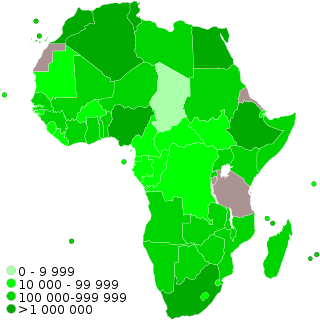
COVID-19 vaccination programs are ongoing in the majority countries and territories in Africa, with 51 of 54 African countries having launched vaccination programs by July 2021. As of October 2023, 51.8% of the continent's population is fully vaccinated with over 1084.5 million doses administered.

The COVID-19 vaccination in Vietnam is an ongoing immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in response to the ongoing pandemic in the country. Following the approval of the Oxford–AstraZeneca COVID-19 vaccine on 30 January 2021, vaccinations commenced on 8 March 2021, and will continue throughout the year with the goal of vaccinating 80% of the population by June 2022. The Sputnik V was later approved for use on 23 March 2021. The Sinopharm BIBP vaccine was approved for emergency use on 4 June 2021, while Pfizer–BioNTech COVID-19 vaccine, Moderna COVID-19 vaccine and Janssen COVID-19 vaccine were approved on 12 June 2021, 29 June 2021, and 15 July 2021, respectively. Vietnam approved Abdala vaccine from Center for Genetic Engineering and Biotechnology on 18 September 2021, and Covaxin from Bharat Biotech on 10 November 2021.

COVID-19 vaccination in Mexico is an ongoing immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.

The COVID-19 vaccination in Indonesia is an ongoing mass immunization in response to the COVID-19 pandemic in Indonesia. On 13 January 2021, the program commenced when President Joko Widodo was vaccinated at the presidential palace. In terms of total doses given, Indonesia ranks third in Asia and fifth in the world.
The COVID-19 vaccination program in Argentina is an ongoing effort of mass immunization. Vaccination against COVID-19 began in Argentina on 29 December 2020 aiming at health professionals. Argentina struck a deal with the United Kingdom in November 2020 for a British made vaccine produced by the pharmaceutical company AstraZeneca and the University of Oxford. The vaccines are part of a deal where Argentina received 22.4 million doses. During the first week, 39,599 doses were applied to health professionals.

Bangladesh began the administration of COVID-19 vaccines on 27 January 2021 while mass vaccination started on 7 February 2021.

COVID-19 vaccination in Sri Lanka is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, in response to the ongoing pandemic in the country. As of late July, the Sinopharm BIBP vaccine accounted for 78% of the total 13.8 million vaccines obtained by Sri Lanka to date. The United States donated over 1.5 million Moderna vaccine through COVAX.

COVID-19 vaccination in Taiwan is an ongoing immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in response to the ongoing pandemic in the country.

Skycovione is a COVID-19 vaccine candidate developed by SK Bioscience and the Institute for Protein Design of the University of Washington, It is South Korea's first homegrown COVID-19 vaccine and utilizes GSK's AS03 adjuvant technology.













