The Joint Committee on Vaccination and Immunisation (JCVI) is an independent expert advisory committee that advises United Kingdom health departments on immunisation, making recommendations concerning vaccination schedules and vaccine safety. It has a statutory role in England and Wales, and health departments in Scotland and Northern Ireland may choose to accept its advice.

BioNTech SE is a German biotechnology company based in Mainz that develops and manufactures active immunotherapies for patient-specific approaches to the treatment of diseases. It develops pharmaceutical candidates based on messenger ribonucleic acid (mRNA) for use as individualized cancer immunotherapies, as vaccines against infectious diseases and as protein replacement therapies for rare diseases, and also engineered cell therapy, novel antibodies and small molecule immunomodulators as treatment options for cancer.
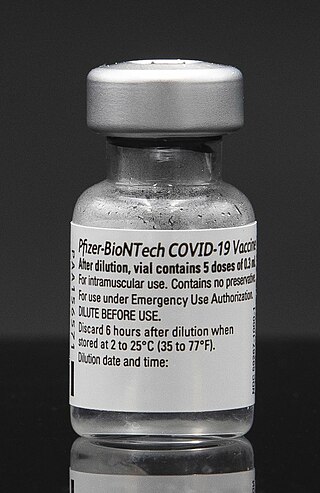
The Pfizer–BioNTech COVID-19 vaccine, sold under the brand name Comirnaty, is an mRNA-based COVID-19 vaccine developed by the German biotechnology company BioNTech. For its development, BioNTech collaborated with the American company Pfizer to carry out clinical trials, logistics, and manufacturing. It is authorized for use in humans to provide protection against COVID-19, caused by infection with the SARS-CoV-2 virus. The vaccine is given by intramuscular injection. It is composed of nucleoside-modified mRNA (modRNA) that encodes a mutated form of the full-length spike protein of SARS-CoV-2, which is encapsulated in lipid nanoparticles. Initial guidance recommended a two-dose regimen, given 21 days apart; this interval was subsequently extended to up to 42 days in the United States, and up to four months in Canada.
The COVID-19 vaccination programme in the United Kingdom is an ongoing mass immunisation campaign for coronavirus disease 2019 (COVID-19) during the COVID-19 pandemic in the United Kingdom.

COVID-19 vaccination in Switzerland is an ongoing immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.

The COVID-19 vaccination campaign in Italy is a mass immunization campaign that was put in place by the Italian government in order to respond to the ongoing COVID-19 pandemic. It started on 27 December 2020, together with most countries in the European Union.

Israel's COVID-19 vaccination programme, officially named "Give a Shoulder", began on 19 December 2020, and has been praised for its speed, having given twenty percent of the Israeli population the first dose of the vaccines' two dose regimen in the span of three weeks.

The general COVID-19 vaccination in Australia program began on 22 February 2021 in response to the COVID-19 pandemic, with the goal of vaccinating all willing people in Australia before 2022. Front-line workers and aged care staff and residents had priority for being inoculated, before a gradual phased release to less-vulnerable and lower-risk population groups throughout 2021. The Therapeutic Goods Administration (TGA) approved four vaccines for Australian use in 2021: the Pfizer–BioNTech vaccine on 25 January, the Oxford–AstraZeneca vaccine on 16 February, Janssen vaccine on 25 June and the Moderna vaccine on 9 August. Although approved for use, the Janssen vaccine was not included in the Australian vaccination program as of June 2021.

COVID-19 vaccination in Canada is an ongoing, intergovernmental effort coordinated between the bodies responsible in the Government of Canada to acquire and distribute vaccines to individual provincial and territorial governments who in turn administer authorized COVID-19 vaccines during the COVID-19 pandemic in Canada. Provinces have worked with local municipal governments, hospital systems, family doctors and independently owned pharmacies to aid in part, or in full with vaccination rollout. The vaccination effort in full is the largest such immunization effort in the nation's history. The vaccination effort began December 14, 2020, and is currently ongoing.
COVID-19 vaccination in Botswana is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.
The COVID-19 vaccination program in Colombia is an ongoing effort of mass immunization put in place by the Colombian government in order to respond to the ongoing COVID-19 pandemic. The virus causing COVID-19 was confirmed to have reached Colombia on 6 March 2020. Colombia's preparation and readiness for a vaccine program allowed it to join the first group of countries who received vaccines through COVAX. The first vaccine in Colombia was given to a nurse on 17 February 2021.

The COVID-19 vaccination in Indonesia is an ongoing mass immunization in response to the COVID-19 pandemic in Indonesia. On 13 January 2021, the program commenced when President Joko Widodo was vaccinated at the presidential palace. In terms of total doses given, Indonesia ranks third in Asia and fifth in the world.

The COVID-19 vaccination in Singapore is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country. Singapore has a very high vaccination rate, with more than 92% of its total population having completed their vaccination regimen.
COVID-19 vaccination in Iceland is an effort to immunize the adult population of Iceland due to the COVID-19 pandemic. As of July 2021, more than 260,000 individuals had received at least one dose of COVID-19 vaccine, which was over 78% of the country's population. On November 21, 2021, 90% of the target population had been fully vaccinated, while around 1 in 5 people had received a booster on top of that; by December 9, 2021, the share of the population having received a booster shot exceeded 50%. On December 13, 2021, the country began offering Pfizer vaccinations to children aged 5–11.

COVID-19 vaccination in Sri Lanka is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, in response to the ongoing pandemic in the country. As of late July, the Sinopharm BIBP vaccine accounted for 78% of the total 13.8 million vaccines obtained by Sri Lanka to date. The United States donated over 1.5 million Moderna vaccine through COVAX.

COVID-19 vaccination in Taiwan is an ongoing immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in response to the ongoing pandemic in the country.
COVID-19 vaccination in Egypt is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.

The COVID-19 vaccination campaign in Ukraine is an ongoing mass immunization campaign for the COVID-19 pandemic in Ukraine.
COVID-19 vaccination in Ontario began in December 2020, when the first doses of the Pfizer vaccine were administered. In February 2021, shipments for both the Pfizer and Moderna vaccines increased significantly. By May 2021, over 50 percent of Ontarians had received their first dose. By the beginning of 2022, over 80 percent of Ontarians had received their first dose.












