
Rubia is the type genus of the Rubiaceae family of flowering plants, which also contains coffee. It contains around 80 species of perennial scrambling or climbing herbs and subshrubs native to the Old World. The genus and its best-known species are commonly known as madder, e.g. Rubia tinctorum, Rubia peregrina, and Rubia cordifolia.

Alizarin is an organic compound with formula C
14H
8O
4 that has been used throughout history as a red dye, principally for dyeing textile fabrics. Historically it was derived from the roots of plants of the madder genus. In 1869, it became the first natural dye to be produced synthetically.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.

Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Several isomers exist but these terms usually refer to 9,10-anthraquinone wherein the keto groups are located on the central ring. It is used as a digester additive to wood pulp for papermaking. Many anthraquinone derivatives are generated by organisms or synthesised industrially for use as dyes, pharmaceuticals, and catalysts. Anthraquinone is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite.

1,2,4-Trihydroxyanthraquinone, commonly called purpurin, is an anthraquinone. It is a naturally occurring red/yellow dye. It is formally derived from 9,10-anthraquinone by replacement of three hydrogen atoms by hydroxyl (OH) groups.

For the parent molecule 9,10-anthraquinone, see anthraquinone

Henry Edward Schunck, also known as Edward von Schunck, was a British chemist who did much work with dyes.
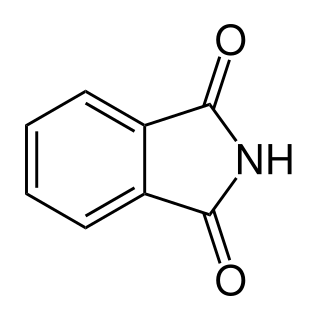
Phthalimide is the organic compound with the formula C6H4(CO)2NH. It is the imide derivative of phthalic anhydride. It is a sublimable white solid that is slightly soluble in water but more so upon addition of base. It is used as a precursor to other organic compounds as a masked source of ammonia.

Rose madder is a red paint made from the pigment madder lake, a traditional lake pigment extracted from the common madder plant Rubia tinctorum.

Rubia cordifolia, known as Indian madder, is a species of flowering plant in the coffee family, Rubiaceae. It has been cultivated for a red pigment derived from roots.

1,3-Dihydroxyanthraquinone, also called purpuroxanthin or xanthopurpurin, is an organic compound with formula C
14H
8O
4 that occurs in the plant Rubia cordifolia. It is one of ten dihydroxyanthraquinone isomers. Its molecular structure can be viewed as being derived from anthraquinone by replacement of two hydrogen atoms (H) by hydroxyl groups (-OH).
A dihydroxyanthraquinone is any of several isomeric organic compounds with formula (C12H62)(CO)2, formally derived from 9,10-anthraquinone by replacing two hydrogen atoms by hydroxyl groups. Dihyroxyantraquinones have been studied since the early 1900s, and include some compounds of historical and current importance. The isomers differ in the position of the hydroxyl groups, and of the carbonyl oxygens (=O) of the underlying anthraquinone.
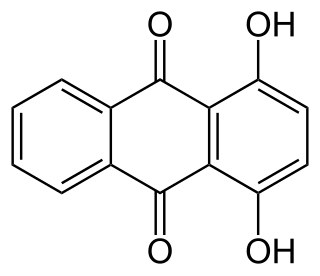
In organic chemistry hydroxyanthraquinones refers to compounds with the formula C12H8-n(OH)n(CO)2 where n ≥ 1. Almost all hydroxyanthraquinones are derivative of 9,10-anthraquinone.
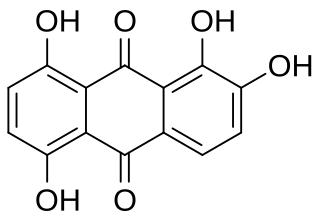
Quinalizarin or 1,2,5,8-tetrahydroxyanthraquinone is an organic compound with formula C12H4(OH)4(CO)2. It is one of many tetrahydroxyanthraquinone isomers, formally derived from anthraquinone by replacement of four hydrogen atoms by hydroxyl (OH) groups at the 1, 2, 5, and 8 positions.

Rubia tinctorum, the rose madder or common madder or dyer's madder, is a herbaceous perennial plant species belonging to the bedstraw and coffee family Rubiaceae.

Anthraquinone dyes are an abundant group of dyes comprising a anthraquinone unit as the shared structural element. Anthraquinone itself is colourless, but red to blue dyes are obtained by introducing electron donor groups such as hydroxy or amino groups in the 1-, 4-, 5- or 8-position. Anthraquinone dyestuffs are structurally related to indigo dyestuffs and are classified together with these in the group of carbonyl dyes.
Aluminium triacetate, formally named aluminium acetate, is a chemical compound with composition Al(CH
3CO
2)
3. Under standard conditions it appears as a white, water-soluble solid that decomposes on heating at around 200 °C. The triacetate hydrolyses to a mixture of basic hydroxide / acetate salts, and multiple species co-exist in chemical equilibrium, particularly in aqueous solutions of the acetate ion; the name aluminium acetate is commonly used for this mixed system.
4-Chlorophenol is an organic compound with the formula C6H4ClOH. It is one of three monochlorophenol isomers. It is a colorless or white solid that melts easily and exhibits significant solubility in water. Its pKa is 9.14.

2-Methylanthraquinone, also known as β-methylanthraquinone and tectoquinone, is an organic compound which is a methylated derivative of anthraquinone. An off-white solid, it is an important precursor to many dyes. It is present in the wood of the teak tree, where it gives the tree resistance to insects.
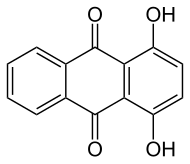

![Summary equation for one synthesis of alizarin.[sic.] QuinizarinPrep.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/2/23/QuinizarinPrep.svg/444px-QuinizarinPrep.svg.png)
















