
Empathogens or entactogens are a class of psychoactive drugs that induce the production of experiences of emotional communion, oneness, relatedness, emotional openness—that is, empathy or sympathy—as particularly observed and reported for experiences with 3,4-methylenedioxymethamphetamine (MDMA). This class of drug is distinguished from the classes of hallucinogen or psychedelic, and amphetamine or stimulants. Major members of this class include MDMA, MDA, MDEA, MDOH, MBDB, 5-APB, 5-MAPB, 6-APB, 6-MAPB, methylone, mephedrone, GHB, αMT, and αET, MDAI among others. Most entactogens are phenethylamines and amphetamines, although several, such as αMT and αET, are tryptamines. When referring to MDMA and its counterparts, the term MDxx is often used. Entactogens are sometimes incorrectly referred to as hallucinogens or stimulants, although many entactogens such as ecstasy exhibit psychedelic or stimulant properties as well.

4-Methylaminorex is a stimulant drug of the 2-amino-5-aryloxazoline class that was first synthesized in 1960 by McNeil Laboratories. It is also known by its street name "U4Euh" ("Euphoria"). It is banned in many countries as a stimulant.

Tyrosine hydroxylase or tyrosine 3-monooxygenase is the enzyme responsible for catalyzing the conversion of the amino acid L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA). It does so using molecular oxygen (O2), as well as iron (Fe2+) and tetrahydrobiopterin as cofactors. L-DOPA is a precursor for dopamine, which, in turn, is a precursor for the important neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline). Tyrosine hydroxylase catalyzes the rate limiting step in this synthesis of catecholamines. In humans, tyrosine hydroxylase is encoded by the TH gene, and the enzyme is present in the central nervous system (CNS), peripheral sympathetic neurons and the adrenal medulla. Tyrosine hydroxylase, phenylalanine hydroxylase and tryptophan hydroxylase together make up the family of aromatic amino acid hydroxylases (AAAHs).
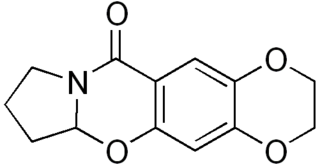
CX-614 is an ampakine drug developed by Cortex Pharmaceuticals. It has been investigated for its effect on AMPA receptors.

In enzymology, a kynurenine 3-monooxygenase (EC 1.14.13.9) is an enzyme that catalyzes the chemical reaction

Tryptophan hydroxylase 2 (TPH2) is an isozyme of tryptophan hydroxylase found in vertebrates. In humans, TPH2 is primarily expressed in the serotonergic neurons of the brain, with the highest expression in the raphe nucleus of the midbrain. Until the discovery of TPH2 in 2003, serotonin levels in the central nervous system were believed to be regulated by serotonin synthesis in peripheral tissues, in which tryptophan hydroxylase is the dominant form.
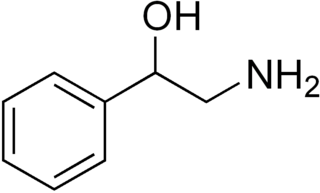
Phenylethanolamine, or β-hydroxyphenethylamine, is a trace amine with a structure similar to those of other trace phenethylamines as well as the catecholamine neurotransmitters dopamine, norepinephrine, and epinephrine. As an organic compound, phenylethanolamine is a β-hydroxylated phenethylamine that is also structurally related to a number of synthetic drugs in the substituted phenethylamine class. In common with these compounds, phenylethanolamine has strong cardiovascular activity and, under the name Apophedrin, has been used as a drug to produce topical vasoconstriction.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of one or more monoamine neurotransmitters from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitters and hence enhanced signaling by those neurotransmitters. The monoamine neurotransmitters include serotonin, norepinephrine, and dopamine; monoamine releasing agents can induce the release of one or more of these neurotransmitters.
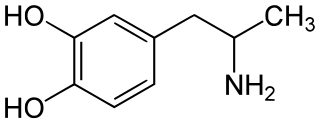
α-Methyldopamine (α-Me-DA), also known as 3,4-dihydroxyamphetamine or as catecholamphetamine, is a research chemical of the catecholamine and amphetamine families. It is a monoamine releasing agent and a metabolite of MDMA and MDA. The bis-glutathionyl metabolite of α-methyldopamine is slightly neurotoxic when directly injected into the brain's ventricles.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

N,N-Dimethyldopamine (DMDA) is an organic compound belonging to the phenethylamine family. It is related structurally to the alkaloid epinine (N-methyldopamine) and to the major neurotransmitter dopamine (of which it is the N,N-dimethylated analog). Because of its structural relationship to dopamine, DMDA has been the subject of a number of pharmacological investigations. DMDA has been detected in Acacia rigidula.

4-Hydroxy-3-methoxymethamphetamine (HMMA) is an active metabolite of 3,4-methylenedioxymethamphetamine (MDMA). It is a slightly more potent stimulant than MDMA in rodents. The drug is substantially less potent than MDMA as a monoamine releasing agent in vitro. Nonetheless, HMMA has been found to induce the release of serotonin, norepinephrine, and dopamine with EC50Tooltip half-maximal effective concentration values of 589 nM, 625 nM, and 607–2884 nM, respectively, and hence acts as a lower-potency serotonin–norepinephrine–dopamine releasing agent (SNDRA). The predicted log P of HMMA is 1.2.

5-Chloro-α-methyltryptamine (5-Chloro-αMT), also known as PAL-542, is a tryptamine derivative related to α-methyltryptamine (αMT) and one of only a few known specific serotonin-dopamine releasing agents (SDRAs). It has been investigated in animals as a potential treatment for cocaine dependence. The EC50 values of 5-chloro-αMT in evoking the in vitro release of serotonin (5-HT), dopamine (DA), and norepinephrine (NE) in rat synaptosomes were reported as 16 nM, 54 nM, and 3434 nM, with an NE/DA ratio of 63.6 and a DA/5-HT ratio of 3.38, indicating that it is a highly specific and well-balanced SDRA. However, 5-chloro-αMT has also been found to act as a potent full agonist of the 5-HT2A receptor, with an EC50 value of 6.27 nM and an efficacy of 105%. It is likely to act as a potent agonist of other serotonin receptors as well.

2,4,5-Trihydroxymethamphetamine is a neurotoxin and a metabolite of MDMA. It has structural similarity to the dopamine neurotoxin 6-hydroxydopamine, and produces lasting serotonin deficits when administered centrally.

A monoamine neurotoxin, or monoaminergic neurotoxin, is a drug that selectively damages or destroys monoaminergic neurons. Monoaminergic neurons are neurons that signal via stimulation by monoamine neurotransmitters including serotonin, dopamine, and norepinephrine.

3,4-Dihydroxymethamphetamine, or 3,4-dihydroxy-N-methylamphetamine, also known as α-methylepinine or α,N-dimethyldopamine, is the major metabolite of 3,4-methylenedioxy-N-methylamphetamine (MDMA). It is formed from MDMA by O-demethylation via cytochrome P450 enzymes including CYP2D6 as well as CYP1A2 and CYP3A4. Like MDMA, HHMA is a monoamine releasing agent.

α-Methyltryptophan is a synthetic tryptamine derivative, an artificial amino acid, and a prodrug of α-methylserotonin (αMS). It is the α-methylated derivative of tryptophan, while αMS is the α-methylated analogue of serotonin. αMTP has been suggested for potential therapeutic use in the treatment of conditions thought by some authors to be related to serotonin deficiency, such as depression. In labeled forms, αMTP is also used as a radiotracer in positron emission tomography (PET) imaging to assess serotonin synthesis and certain other processes.

α-Methylphenylalanine is an artificial amino acid and a phenethylamine and amphetamine derivative. It is the α-methylated analogue of phenylalanine, the precursor of the catecholamine neurotransmitters, and the amino acid analogue of amphetamine (α-methylphenethylamine), a psychostimulant and monoamine releasing agent.

α-Methyl-5-hydroxytryptophan (α-Me-5-HTP) is a synthetic tryptamine derivative, an artificial amino acid, and a prodrug of α-methylserotonin. It is the α-methylated derivative of 5-hydroxytryptophan (5-HTP), while αMS is the α-methylated analogue of serotonin. Along with α-methyltryptophan (α-MTP), α-Me-5-HTP has been suggested for potential therapeutic use in the treatment of conditions thought by some authors to be related to serotonin deficiency, such as depression.

4-Hydroxy-3-methoxyamphetamine (HMA), also known as 3-O-methyl-α-methyldopamine, is an active metabolite of 3,4-methylenedioxymethamphetamine (MDMA). It is substantially less potent than MDMA or 3,4-methylenedioxyamphetamine (MDA) as a monoamine releasing agent in vitro. Nonetheless, HMA has been found to induce the release of serotonin, norepinephrine, and dopamine with EC50Tooltip half-maximal effective concentration values of 897 nM, 694 nM, and 1450–3423 nM, respectively, and hence acts as a lower-potency serotonin–norepinephrine–dopamine releasing agent (SNDRA). The predicted log P of HMA is 0.6.



















