In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.
Acrylonitrile is an organic compound with the formula CH2CHCN and the structure H2C=CH−C≡N. It is a colorless, volatile liquid. It has a pungent odor of garlic or onions. Its molecular structure consists of a vinyl group linked to a nitrile. It is an important monomer for the manufacture of useful plastics such as polyacrylonitrile. It is reactive and toxic at low doses.

1,3-Butadiene is the organic compound with the formula CH2=CH-CH=CH2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene.
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At its simplest, it's a useful way of converting alkanes, which are relatively inert and thus low-valued, to olefins, which are reactive and thus more valuable. Alkenes are precursors to aldehydes, alcohols, polymers, and aromatics. As a problematic reaction, the fouling and inactivation of many catalysts arises via coking, which is the dehydrogenative polymerization of organic substrates.
In chemistry, a trimer is a molecule or polyatomic anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer derived from three identical precursors often in competition with polymerization.
IARC group 2B substances, mixtures and exposure circumstances are those that have been classified as "possibly carcinogenic to humans" by the International Agency for Research on Cancer (IARC) as This category is used when there is limited evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals. It may also be used when there is inadequate evidence of carcinogenicity in humans but there is sufficient evidence of carcinogenicity in experimental animals. In some instances, an agent, mixture or exposure circumstance for which there is inadequate evidence of carcinogenicity in humans, but limited evidence of carcinogenicity in experimental animals together with supporting evidence from other relevant data may be placed in this group.
Thailand's Psychotropic Substances Act is a law designed to regulate certain mind-altering drugs. According to the Office of the Narcotics Control Board, "The Act directly resulted from the Convention on Psychotropic Substances 1971 of which Thailand is a party." The Act divides psychotropic drugs into four Schedules. Offenses involving Schedule I and II drugs carry heavier penalties than those involving Schedule III and IV drugs. Note that this statute does not regulate most opioids, cocaine, or some amphetamines. The vast majority of narcotic painkillers, along with cocaine and most amphetamines are regulated under the Narcotics Act.

Vinylacetylene is the organic compound with the formula C4H4. The colourless gas was once used in the polymer industry. It is composed of both alkyne and alkene groups and is the simplest enyne.

Adiponitrile is an organic compound with the chemical formula (CH2)4(CN)2. This viscous, colourless dinitrile is an important precursor to the polymer nylon 66. In 2005, about one million tonnes of adiponitrile were produced.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 CFR 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.
In electrochemistry, electrosynthesis is the synthesis of chemical compounds in an electrochemical cell. Compared to ordinary redox reactions, electrosynthesis sometimes offers improved selectivity and yields. Electrosynthesis is actively studied as a science and also has industrial applications. Electrooxidation has potential for wastewater treatment as well.

Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source of butadiene.

Methyl acrylate is an organic compound, more accurately the methyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced to make acrylate fiber, which is used to weave synthetic carpets. It is also a reagent in the synthesis of various pharmaceutical intermediates. Owing to the tendency of methyl acrylate to polymerize, samples typically contain an inhibitor such as hydroquinone.
In nitrile reduction a nitrile is reduced to either an amine or an aldehyde with a suitable chemical reagent.
Nitrile anions is jargon from the organic product resulting from the deprotonation of alkylnitriles. The proton(s) α to the nitrile group are sufficiently acidic that they undergo deprotonation by strong bases, usually lithium-derived. The products are not anions but covalent organolithium complexes. Regardless, these organolithium compounds are reactive toward various electrophiles.
Methacrylonitrile, MeAN in short, is a chemical compound that is an unsaturated aliphatic nitrile, widely used in the preparation of homopolymers, copolymers, elastomers, and plastics and as a chemical intermediate in the preparation of acids, amides, amines, esters, and other nitriles. MeAN is also used as a replacement for acrylonitrile in the manufacture of an acrylonitrile/butadiene/styrene-like polymer. It is a clear and colorless liquid, that has a bitter almond smell.

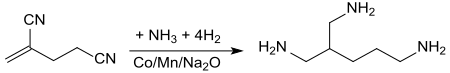
2-Methylglutaronitrile is the organic compound with the formula NCCH2CH2CH(CH3)CN. This dinitrile is obtained in the large-scale synthesis of adiponitrile. It is a colorless liquid with an unpleasant odor. It is the starting compound for the vitamin nicotinamide and for the diester dimethyl-2-methylglutarate and the ester amide methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate, which are promoted as green solvents. 2-Methylglutaronitrile is chiral but is mainly encountered as the racemate. It is also used to make Dytek A.

Nitrilotriacetonitrile (NTAN) is a precursor for nitrilotriacetic acid, for tris(2-aminoethyl)amine and for the epoxy resin crosslinker aminoethylpiperazine.
1-Vinylimidazole is a water-soluble basic monomer that forms quaternizable homopolymers by free-radical polymerization with a variety of vinyl and acrylic monomers. The products are functional copolymers, which are used as oil field chemicals and as cosmetic auxiliaries. 1-Vinylimidazole acts as a reactive diluent in UV lacquers, inks, and adhesives.