Antimalarial medications or simply antimalarials are a type of antiparasitic chemical agent, often naturally derived, that can be used to treat or to prevent malaria, in the latter case, most often aiming at two susceptible target groups, young children and pregnant women. As of 2018, modern treatments, including for severe malaria, continued to depend on therapies deriving historically from quinine and artesunate, both parenteral (injectable) drugs, expanding from there into the many classes of available modern drugs. Incidence and distribution of the disease is expected to remain high, globally, for many years to come; moreover, known antimalarial drugs have repeatedly been observed to elicit resistance in the malaria parasite—including for combination therapies featuring artemisinin, a drug of last resort, where resistance has now been observed in Southeast Asia. As such, the needs for new antimalarial agents and new strategies of treatment remain important priorities in tropical medicine. As well, despite very positive outcomes from many modern treatments, serious side effects can impact some individuals taking standard doses.
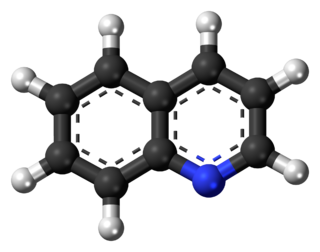
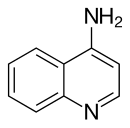
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

Porphyria cutanea tarda is the most common subtype of porphyria. The disease is named because it is a porphyria that often presents with skin manifestations later in life. The disorder results from low levels of the enzyme responsible for the fifth step in heme production. Heme is a vital molecule for all of the body's organs. It is a component of hemoglobin, the molecule that carries oxygen in the blood.
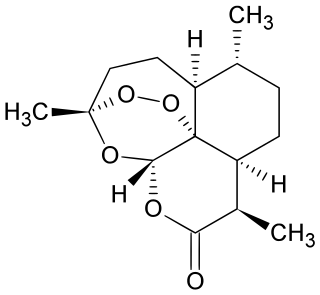
Artemisinin and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to Plasmodium falciparum. It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her discovery. Artemisinin-based combination therapies (ACTs) are now standard treatment worldwide for P. falciparum malaria as well as malaria due to other species of Plasmodium. Artemisinin is extracted from the plant Artemisia annua an herb employed in Chinese traditional medicine. A precursor compound can be produced using a genetically engineered yeast, which is much more efficient than using the plant.

Mepacrine, also called quinacrine or by the trade names Atabrine or Atebrin, is a medication with several uses. It is related to chloroquine and mefloquine. Although available from compounding pharmacies, as of August 2020 approved formulations are not available in the United States.
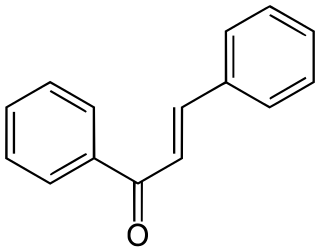
Chalcone is the organic compound C6H5C(O)CH=CHC6H5. It is an α,β-unsaturated ketone. A variety of important biological compounds are known collectively as chalcones or chalconoids. They are widely known bioactive substances, fluorescent materials, and chemical intermediates.
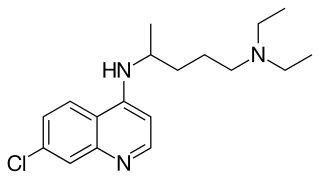
Chloroquine is an antiparasitic medication that treats malaria. It works by increasing the levels of haeme in the blood, a substance toxic to the malarial parasite. This kills the parasite and stops the infection from spreading. Certain types of malaria, resistant strains, and complicated cases typically require different or additional medication. Chloroquine is also occasionally used for amebiasis that is occurring outside the intestines, rheumatoid arthritis, and lupus erythematosus. While it has not been formally studied in pregnancy, it appears safe. It was studied to treat COVID-19 early in the pandemic, but these studies were largely halted in the northern summer of 2020, and the NIH does not recommend its use for this purpose. It is taken by mouth.
Quinazoline is an organic compound with the formula C8H6N2. It is an aromatic heterocycle with a bicyclic structure consisting of two fused six-membered aromatic rings, a benzene ring and a pyrimidine ring. It is a light yellow crystalline solid that is soluble in water. Also known as 1,3-diazanaphthalene, quinazoline received its name from being an aza derivative of quinoline. Though the parent quinazoline molecule is rarely mentioned by itself in technical literature, substituted derivatives have been synthesized for medicinal purposes such as antimalarial and anticancer agents. Quinazoline is a planar molecule. It is isomeric with the other diazanaphthalenes of the benzodiazine subgroup: cinnoline, quinoxaline, and phthalazine. Over 200 biologically active quinazoline and quinoline alkaloids are identified.

Hydroxychloroquine, sold under the brand name Plaquenil among others, is a medication used to prevent and treat malaria in areas where malaria remains sensitive to chloroquine. Other uses include treatment of rheumatoid arthritis, lupus, and porphyria cutanea tarda. It is taken by mouth, often in the form of hydroxychloroquine sulfate.
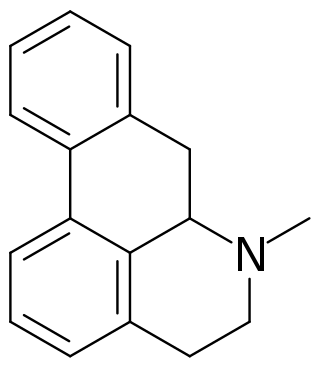
Aporphine is an alkaloid with the chemical formula C17H17N. It is the core chemical substructure of the aporphine alkaloids, a subclass of quinoline alkaloids. It can exist in either of two enantiomeric forms, (R)-aporphine and (S)-aporphine.
The Combes quinoline synthesis is a chemical reaction, which was first reported by Combes in 1888. Further studies and reviews of the Combes quinoline synthesis and its variations have been published by Alyamkina et al., Bergstrom and Franklin, Born, and Johnson and Mathews.

8-Aminoquinoline is the 8-amino derivative of quinoline. Often abbreviated AQ, it is a pale yellow solid. It is structurally analogous to 8-hydroxyquinoline.
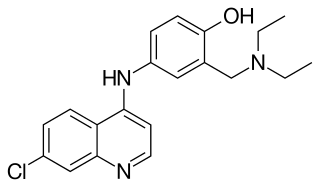
Amodiaquine (ADQ) is a medication used to treat malaria, including Plasmodium falciparum malaria when uncomplicated. It is recommended to be given with artesunate to reduce the risk of resistance. Due to the risk of rare but serious side effects, it is not generally recommended to prevent malaria. Though, the World Health Organization (WHO) in 2013 recommended use for seasonal preventive in children at high risk in combination with sulfadoxine and pyrimethamine.
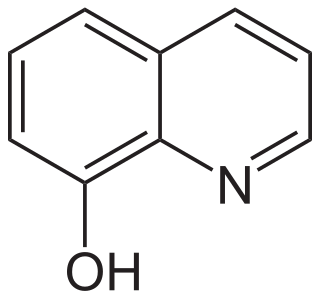
8-Hydroxyquinoline is an organic compound derived from the heterocycle quinoline. A colorless solid, its conjugate base is a chelating agent, which is used for the quantitative determination of metal ions.

The Gould–Jacobs reaction is an organic synthesis for the preparation of quinolines and 4‐hydroxyquinoline derivatives. The Gould–Jacobs reaction is a series of reactions. The series of reactions begins with the condensation/substitution of an aniline with alkoxy methylenemalonic ester or acyl malonic ester, producing anilidomethylenemalonic ester. Then through a 6 electron cyclization process, 4-hydroxy-3-carboalkoxyquinoline is formed, which exist mostly in the 4-oxo form. Saponification results in the formation of an acid. This step is followed by decarboxylation to give 4-hydroxyquinoline. The Gould–Jacobs reaction is effective for anilines with electron‐donating groups at the meta‐position.
Drug-induced pruritus is itchiness of the skin caused by medication, a pruritic reaction that is generalized.

Tenuazonic acid is a mycotoxin produced by Alternaria species. It is a powerful eukaryotic protein synthesis inhibitor. It is a tetrameric acid that is ubiquitous in biological environments and prevents the release of newly synthesized protein from the ribosome. Its toxicity is the highest among all Alternaria mycotoxins and has both phytotoxic and cytotoxic properties. In 1991 Tenuazonic acid was reported to inhibit skin tumor promotion in mice.
Project 523 is a code name for a 1967 secret military project of the People's Republic of China to find antimalarial medications. Named after the date the project launched, 23 May, it addressed malaria, an important threat in the Vietnam War. At the behest of Ho Chi Minh, Prime Minister of North Vietnam, Zhou Enlai, the Premier of the People's Republic of China, convinced Mao Zedong, Chairman of the Chinese Communist Party, to start the mass project "to keep [the] allies' troops combat-ready", as the meeting minutes put it. More than 500 Chinese scientists were recruited. The project was divided into three streams. The one for investigating traditional Chinese medicine discovered and led to the development of a class of new antimalarial drugs called artemisinins. Launched during and lasting throughout the Cultural Revolution, Project 523 was officially terminated in 1981.

4,7-Dichloroquinoline is a two-ring heterocyclic compound used as a chemical intermediate to aminoquinoline antimalarial drugs including amodiaquine, chloroquine and hydroxychloroquine.

Ferroquine is a synthetic compound related to chloroquine which acts as an antimalarial, and shows good activity against chloroquine-resistant strains. It contains an organometallic ferrocene ring which is unusual in pharmaceuticals, and while it was first reported in 1997, it has progressed slowly through clinical trials, with results from Phase II trials showing reasonable safety and efficacy, and further trials ongoing.