Malaria is a mosquito-borne infectious disease that affects vertebrates and Anopheles mosquitoes. Human malaria causes symptoms that typically include fever, fatigue, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. Symptoms usually begin 10 to 15 days after being bitten by an infected Anopheles mosquito. If not properly treated, people may have recurrences of the disease months later. In those who have recently survived an infection, reinfection usually causes milder symptoms. This partial resistance disappears over months to years if the person has no continuing exposure to malaria. The mosquito vector is itself harmed by Plasmodium infections, causing reduced lifespan.

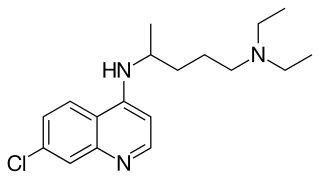
Mefloquine, sold under the brand name Lariam among others, is a medication used to prevent or treat malaria. When used for prevention it is typically started before potential exposure and continued for several weeks after potential exposure. It can be used to treat mild or moderate malaria but is not recommended for severe malaria. It is taken by mouth.
Antimalarial medications or simply antimalarials are a type of antiparasitic chemical agent, often naturally derived, that can be used to treat or to prevent malaria, in the latter case, most often aiming at two susceptible target groups, young children and pregnant women. As of 2018, modern treatments, including for severe malaria, continued to depend on therapies deriving historically from quinine and artesunate, both parenteral (injectable) drugs, expanding from there into the many classes of available modern drugs. Incidence and distribution of the disease is expected to remain high, globally, for many years to come; moreover, known antimalarial drugs have repeatedly been observed to elicit resistance in the malaria parasite—including for combination therapies featuring artemisinin, a drug of last resort, where resistance has now been observed in Southeast Asia. As such, the needs for new antimalarial agents and new strategies of treatment remain important priorities in tropical medicine. As well, despite very positive outcomes from many modern treatments, serious side effects can impact some individuals taking standard doses.

Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans. The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. P. falciparum is therefore regarded as the deadliest parasite in humans. It is also associated with the development of blood cancer and is classified as a Group 2A (probable) carcinogen.
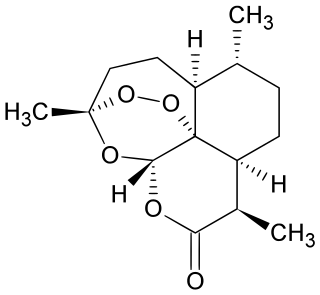
Artemisinin and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to Plasmodium falciparum. It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her discovery. Artemisinin-based combination therapies (ACTs) are now standard treatment worldwide for P. falciparum malaria as well as malaria due to other species of Plasmodium. Artemisinin is extracted from the plant Artemisia annua an herb employed in Chinese traditional medicine. A precursor compound can be produced using a genetically engineered yeast, which is much more efficient than using the plant.

Travel medicine or emporiatrics is the branch of medicine that deals with the prevention and management of health problems of international travelers.
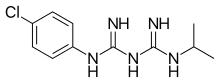
Chloroquine is an antiparasitic medication that treats malaria. It works by increasing the levels of haeme in the blood, a substance toxic to the malarial parasite. This kills the parasite and stops the infection from spreading. Certain types of malaria, resistant strains, and complicated cases typically require different or additional medication. Chloroquine is also occasionally used for amebiasis that is occurring outside the intestines, rheumatoid arthritis, and lupus erythematosus. While it has not been formally studied in pregnancy, it appears safe. It was studied to treat COVID-19 early in the pandemic, but these studies were largely halted in the northern summer of 2020, and the NIH does not recommend its use for this purpose. It is taken by mouth.

Primaquine is a medication used to treat and prevent malaria and to treat Pneumocystis pneumonia. Specifically it is used for malaria due to Plasmodium vivax and Plasmodium ovale along with other medications and for prevention if other options cannot be used. It is an alternative treatment for Pneumocystis pneumonia together with clindamycin. It is taken by mouth.

Plasmodium vivax is a protozoal parasite and a human pathogen. This parasite is the most frequent and widely distributed cause of recurring malaria. Although it is less virulent than Plasmodium falciparum, the deadliest of the five human malaria parasites, P. vivax malaria infections can lead to severe disease and death, often due to splenomegaly. P. vivax is carried by the female Anopheles mosquito; the males do not bite.

Plasmodium ovale is a species of parasitic protozoon that causes tertian malaria in humans. It is one of several species of Plasmodium parasites that infect humans, including Plasmodium falciparum and Plasmodium vivax which are responsible for most cases of malaria in the world. P. ovale is rare compared to these two parasites, and substantially less dangerous than P. falciparum.
Artemether/lumefantrine, sold under the trade name Coartem among others, is a combination of the two medications artemether and lumefantrine. It is used to treat malaria caused by Plasmodium falciparum that is not treatable with chloroquine. It is not typically used to prevent malaria. It is taken by mouth.
Malaria prophylaxis is the preventive treatment of malaria. Several malaria vaccines are under development.
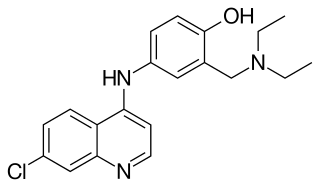
Amodiaquine (ADQ) is a medication used to treat malaria, including Plasmodium falciparum malaria when uncomplicated. It is recommended to be given with artesunate to reduce the risk of resistance. Due to the risk of rare but serious side effects, it is not generally recommended to prevent malaria. Though, the World Health Organization (WHO) in 2013 recommended use for seasonal preventive in children at high risk in combination with sulfadoxine and pyrimethamine.

Cycloguanil is a dihydrofolate reductase inhibitor, and is a metabolite of the antimalarial drug proguanil; its formation in vivo has been thought to be primarily responsible for the antimalarial activity of proguanil. However, more recent work has indicated that, while proguanil is synergistic with the drug atovaquone, cycloguanil is in fact antagonistic to the effects of atovaquone, suggesting that, unlike cycloguanil, proguanil may have an alternative mechanism of antimalarial action besides dihydrofolate reductase inhibition.

The history of malaria extends from its prehistoric origin as a zoonotic disease in the primates of Africa through to the 21st century. A widespread and potentially lethal human infectious disease, at its peak malaria infested every continent except Antarctica. Its prevention and treatment have been targeted in science and medicine for hundreds of years. Since the discovery of the Plasmodium parasites which cause it, research attention has focused on their biology as well as that of the mosquitoes which transmit the parasites.
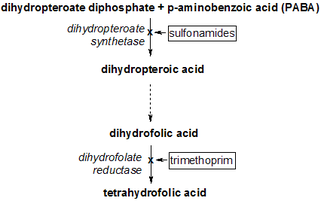
A dihydrofolate reductase inhibitor is a molecule that inhibits the function of dihydrofolate reductase, and is a type of antifolate.

The administration of drugs to whole populations irrespective of disease status is referred to as mass drug administration (MDA) or mass dispensing.
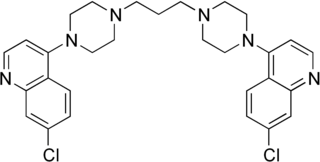
Piperaquine is an antiparasitic drug used in combination with dihydroartemisinin to treat malaria. Piperaquine was developed under the Chinese National Malaria Elimination Programme in the 1960s and was adopted throughout China as a replacement for the structurally similar antimalarial drug chloroquine. Due to widespread parasite resistance to piperaquine, the drug fell out of use as a monotherapy, and is instead used as a partner drug for artemisinin combination therapy. Piperaquine kills parasites by disrupting the detoxification of host heme.
Atovaquone/proguanil, sold under the brand name Malarone among others, is a fixed-dose combination medication used to treat and prevent malaria, including chloroquine-resistant malaria. It contains atovaquone and proguanil. It is not recommended for severe or complicated malaria. It is taken by mouth.
Pregnancy-associated malaria (PAM) or placental malaria is a presentation of malaria in pregnancy which is life-threatening to both pregnant women and unborn fetuses. PAM occurs when a pregnant woman contracts malaria, generally as a result of Plasmodium falciparum infection, and because she is pregnant, is at greater risk of associated complications such as placental malaria. Placental malaria interferes with the transmission of vital substances through the fetal placenta, which can result in stillbirths, miscarriages, and dangerously low birth weights.