Antimalarial medications or simply antimalarials are a type of antiparasitic chemical agent, often naturally derived, that can be used to treat or to prevent malaria, in the latter case, most often aiming at two susceptible target groups, young children and pregnant women. As of 2018, modern treatments, including for severe malaria, continued to depend on therapies deriving historically from quinine and artesunate, both parenteral (injectable) drugs, expanding from there into the many classes of available modern drugs. Incidence and distribution of the disease is expected to remain high, globally, for many years to come; moreover, known antimalarial drugs have repeatedly been observed to elicit resistance in the malaria parasite—including for combination therapies featuring artemisinin, a drug of last resort, where resistance has now been observed in Southeast Asia. As such, the needs for new antimalarial agents and new strategies of treatment remain important priorities in tropical medicine. As well, despite very positive outcomes from many modern treatments, serious side effects can impact some individuals taking standard doses.

Atorvastatin is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a first-line treatment. It is taken by mouth.
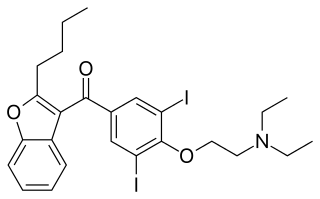
Amiodarone is an antiarrhythmic medication used to treat and prevent a number of types of cardiac dysrhythmias. This includes ventricular tachycardia, ventricular fibrillation, and wide complex tachycardia, atrial fibrillation, and paroxysmal supraventricular tachycardia. Evidence in cardiac arrest, however, is poor. It can be given by mouth, intravenously, or intraosseously. When used by mouth, it can take a few weeks for effects to begin.

Cytochrome P450 3A4 is an important enzyme in the body, mainly found in the liver and in the intestine, which in humans is encoded by CYP3A4 gene. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme.

In pharmaceutical sciences, drug interactions occur when a drug's mechanism of action is affected by the concomitant administration of substances such as foods, beverages, or other drugs. A popular example of drug–food interaction is the effect of grapefruit on the metabolism of drugs.

Artemether is a medication used for the treatment of malaria. The injectable form is specifically used for severe malaria rather than quinine. In adults, it may not be as effective as artesunate. It is given by injection in a muscle. It is also available by mouth in combination with lumefantrine, known as artemether/lumefantrine.
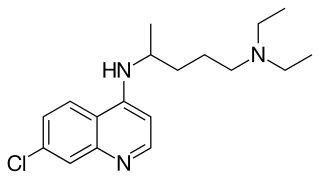
Chloroquine is an antiparasitic medication that treats malaria. It works by increasing the levels of haeme in the blood, a substance toxic to the malarial parasite. This kills the parasite and stops the infection from spreading. Certain types of malaria, resistant strains, and complicated cases typically require different or additional medication. Chloroquine is also occasionally used for amebiasis that is occurring outside the intestines, rheumatoid arthritis, and lupus erythematosus. While it has not been formally studied in pregnancy, it appears safe. It was studied to treat COVID-19 early in the pandemic, but these studies were largely halted in the northern summer of 2020, and the NIH does not recommend its use for this purpose. It is taken by mouth.

Primaquine is a medication used to treat and prevent malaria and to treat Pneumocystis pneumonia. Specifically it is used for malaria due to Plasmodium vivax and Plasmodium ovale along with other medications and for prevention if other options cannot be used. It is an alternative treatment for Pneumocystis pneumonia together with clindamycin. It is taken by mouth.

Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer. This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia. It is often used together with other chemotherapy agents. Doxorubicin is given by injection into a vein.

Proguanil, also known as chlorguanide and chloroguanide, is a medication used to treat and prevent malaria. It is often used together with chloroquine or atovaquone. When used with chloroquine the combination will treat mild chloroquine resistant malaria. It is taken by mouth.

Hydroxychloroquine, sold under the brand name Plaquenil among others, is a medication used to prevent and treat malaria in areas where malaria remains sensitive to chloroquine. Other uses include treatment of rheumatoid arthritis, lupus, and porphyria cutanea tarda. It is taken by mouth, often in the form of hydroxychloroquine sulfate.

Naringin is a flavanone-7-O-glycoside between the flavanone naringenin and the disaccharide neohesperidose. The flavonoid naringin occurs naturally in citrus fruits, especially in grapefruit, where naringin is responsible for the fruit's bitter taste. In commercial grapefruit juice production, the enzyme naringinase can be used to remove the bitterness (debittering) created by naringin. In humans naringin is metabolized to the aglycone naringenin by naringinase present in the gut.
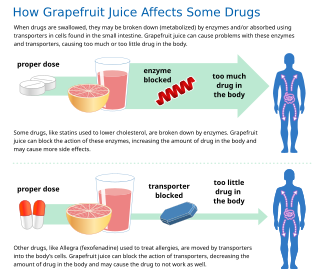
Some fruit juices and fruits can interact with numerous drugs, in many cases causing adverse effects. The effect is most studied with grapefruit and grapefruit juice, but similar effects have been observed with certain other citrus fruits.
Artemether/lumefantrine, sold under the trade name Coartem among others, is a combination of the two medications artemether and lumefantrine. It is used to treat malaria caused by Plasmodium falciparum that is not treatable with chloroquine. It is not typically used to prevent malaria. It is taken by mouth.
Synergistic enhancers of antiretrovirals usually do not possess any antiretroviral properties alone, but when they are taken concurrently with antiretroviral drugs they enhance the effect of that drug.

Bergamottin (5-geranoxypsoralen) is a natural furanocoumarin found in the pulp of pomelos and grapefruits. It is also found in the peel and pulp of the bergamot orange, from which it was first isolated and from which its name is derived.

Haemozoin is a disposal product formed from the digestion of blood by some blood-feeding parasites. These hematophagous organisms such as malaria parasites, Rhodnius and Schistosoma digest haemoglobin and release high quantities of free heme, which is the non-protein component of haemoglobin. Heme is a prosthetic group consisting of an iron atom contained in the center of a heterocyclic porphyrin ring. Free heme is toxic to cells, so the parasites convert it into an insoluble crystalline form called hemozoin. In malaria parasites, hemozoin is often called malaria pigment.
Pharmacotoxicology entails the study of the consequences of toxic exposure to pharmaceutical drugs and agents in the health care field. The field of pharmacotoxicology also involves the treatment and prevention of pharmaceutically induced side effects. Pharmacotoxicology can be separated into two different categories: pharmacodynamics, and pharmacokinetics.
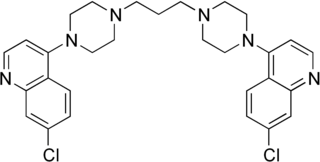
Piperaquine is an antiparasitic drug used in combination with dihydroartemisinin to treat malaria. Piperaquine was developed under the Chinese National Malaria Elimination Programme in the 1960s and was adopted throughout China as a replacement for the structurally similar antimalarial drug chloroquine. Due to widespread parasite resistance to piperaquine, the drug fell out of use as a monotherapy, and is instead used as a partner drug for artemisinin combination therapy. Piperaquine kills parasites by disrupting the detoxification of host heme.

Ganaplacide is a drug in development by Novartis for the purpose of treating malaria. It is a imidazolopiperazine derivative. It has shown activity against the Plasmodium falciparum and Plasmodium vivax forms of the malaria parasite.
















