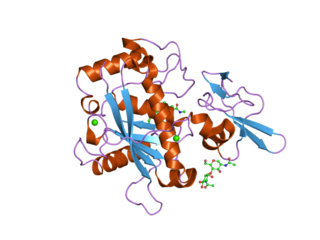
A disintegrin and metalloproteinase with thrombospondin motifs 5 also known as ADAMTS5 is an enzyme that in humans is encoded by the ADAMTS5 gene.

ADAMTS13 —also known as von Willebrand factor-cleaving protease (VWFCP)—is a zinc-containing metalloprotease enzyme that cleaves von Willebrand factor (vWf), a large protein involved in blood clotting. It is secreted into the blood and degrades large vWf multimers, decreasing their activity.
A disintegrin and metalloproteinase with thrombospondin motifs 2 (ADAM-TS2) also known as procollagen I N-proteinase is an enzyme that in humans is encoded by the ADAMTS2 gene.

ADAMs are a family of single-pass transmembrane and secreted metalloendopeptidases. All ADAMs are characterized by a particular domain organization featuring a pro-domain, a metalloprotease, a disintegrin, a cysteine-rich, an epidermal-growth factor like and a transmembrane domain, as well as a C-terminal cytoplasmic tail. Nonetheless, not all human ADAMs have a functional protease domain, which indicates that their biological function mainly depends on protein–protein interactions. Those ADAMs which are active proteases are classified as sheddases because they cut off or shed extracellular portions of transmembrane proteins. For example, ADAM10 can cut off part of the HER2 receptor, thereby activating it. ADAM genes are found in animals, choanoflagellates, fungi and some groups of green algae. Most green algae and all land plants likely lost ADAM proteins.

A disintegrin and metalloproteinase with thrombospondin motifs 4 is an enzyme that in humans is encoded by the ADAMTS4 gene.
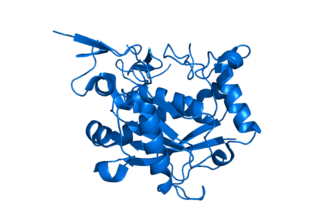
A disintegrin and metalloproteinase with thrombospondin motifs 1 is an enzyme that in humans is encoded by the ADAMTS1 gene.

Disintegrin and metalloproteinase domain-containing protein 9 is an enzyme that in humans is encoded by the ADAM9 gene.

ADAMTS-like protein 4 is a protein that in humans is encoded by the ADAMTSL4 gene.
ADAMTS is a family of multidomain extracellular protease enzymes. 19 members of this family have been identified in humans, the first of which, ADAMTS1, was described in 1997. Known functions of the ADAMTS proteases include processing of procollagens and von Willebrand factor as well as cleavage of aggrecan, versican, brevican and neurocan, making them key remodeling enzymes of the extracellular matrix. They have been demonstrated to have important roles in connective tissue organization, coagulation, inflammation, arthritis, angiogenesis and cell migration. Homologous subfamily of ADAMTSL (ADAMTS-like) proteins, which lack enzymatic activity, has also been described. Most cases of thrombotic thrombocytopenic purpura arise from autoantibody-mediated inhibition of ADAMTS13.

A disintegrin and metalloproteinase with thrombospondin motifs 9 is an enzyme that in humans is encoded by the ADAMTS9 gene.

A disintegrin and metalloproteinase with thrombospondin motifs 10 is an enzyme that in humans is encoded by the ADAMTS10 gene.

ADAMTS-like protein 1 is a protein that in humans is encoded by the ADAMTSL1 gene.

A disintegrin and metalloproteinase with thrombospondin motifs 3 is an enzyme that in humans is encoded by the ADAMTS3 gene. The protein encoded by this gene is the major procollagen II N-propeptidase.

A disintegrin and metalloproteinase with thrombospondin motifs 12 is an enzyme that in humans is encoded by the ADAMTS12 gene.

Collagen and calcium-binding EGF domain-containing protein 1 is a protein that in humans is encoded by the CCBE1 gene.

Papilin is a protein that in humans is encoded by the PAPLN gene. Papilin is an extracellular matrix glycoprotein.
ADAM metallopeptidase with thrombospondin type 1 motif, 17 is a protein that in humans is encoded by the ADAMTS17 gene.
A disintegrin and metalloproteinase with thrombospondin motifs 7 (ADAMTS7) is an enzyme that in humans is encoded by the ADAMTS7 gene on chromosome 15. It is ubiquitously expressed in many tissues and cell types. This enzyme catalyzes the degradation of cartilage oligomeric matrix protein (COMP) degradation. ADAMTS7 has been associated with cancer and arthritis in multiple tissue types. The ADAMTS7 gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.

ADAM metallopeptidase with thrombospondin type 1 motif 6 is a protein that in humans is encoded by the ADAMTS6 gene.
ADAMTS14 encodes a member of the ADAMTS protein family. Members of the family share several distinct protein modules, including a propeptide region, a metalloproteinase domain, a disintegrin-like domain, and a thrombospondin type 1 (TS) motif. Individual members of this family differ in the number of C-terminal TS motifs, and some have unique C-terminal domains. Mature enzyme is generated by the proteolytically process of the encoded preproprotein. The enzyme cleaves the amino-propeptides of fibrillar collagens, enabling collagen fibril formation prior to assembly of collagen, which is a major extracellular matrix (ECM) protein.



















