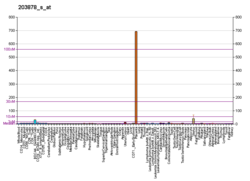
Function
Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. Most MMPs are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. However, the enzyme encoded by this gene is activated intracellularly by furin within the constitutive secretory pathway. Also in contrast to other MMPs, this enzyme cleaves alpha 1-proteinase inhibitor but weakly degrades structural proteins of the extracellular matrix. [7]
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.