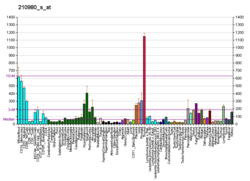
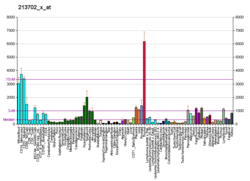
| ASAH1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | ASAH1 , AC, ACDase, ASAH, PHP, PHP32, SMAPME, N-acylsphingosine amidohydrolase (acid ceramidase) 1, N-acylsphingosine amidohydrolase 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 613468; MGI: 1277124; HomoloGene: 10504; GeneCards: ASAH1; OMA:ASAH1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
The ASAH1 gene encodes in humans the acid ceramidase enzyme. [5] [6] [7]