Tetrachloroethylene, also known as perchloroethylene or under the systematic name tetrachloroethene, and abbreviations such as perc, and PCE, is a chlorocarbon with the formula Cl2C=CCl2. It is a non-flammable, stable, colorless and heavy liquid widely used for dry cleaning of fabrics, hence it is sometimes called "dry-cleaning fluid". It also has its uses as an effective automotive brake cleaner. It has a mild sweet, sharp odor, detectable by most people at a concentration of 50 ppm.
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula CH3Cl. One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in industrial chemistry, although it is rarely present in consumer products, and was formerly utilized as a refrigerant. Most chloromethane is biogenic.

Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). Vinyl chloride monomer is among the top twenty largest petrochemicals (petroleum-derived chemicals) in world production. The United States remains the largest vinyl chloride manufacturing region because of its low-production-cost position in chlorine and ethylene raw materials. China is also a large manufacturer and one of the largest consumers of vinyl chloride. Vinyl chloride is a flammable gas that has a sweet odor and is carcinogenic. It can be formed in the environment when soil organisms break down chlorinated solvents. Vinyl chloride that is released by industries or formed by the breakdown of other chlorinated chemicals can enter the air and drinking water supplies. Vinyl chloride is a common contaminant found near landfills. Before the 1970s, vinyl chloride was used as an aerosol propellant and refrigerant.
Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals.

Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene. It is an alkylating agent, which makes it both useful and hazardous to handle.

Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure.

Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and the hemipentahydrate CdCl2•2.5H2O.

Chloroprene (IUPAC name 2-chlorobuta-1,3-diene) is a chemical compound with the molecular formula CH2=CCl−CH=CH2. Chloroprene is a colorless volatile liquid, almost exclusively used as a monomer for the production of the polymer polychloroprene, better known as neoprene, a type of synthetic rubber.

Chlorobenzene (abbreviated PhCl) is an aryl chloride and the simplest of the chlorobenzenes, consisting of a benzene ring substituted with one chlorine atom. Its chemical formula is C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water and other chlorocarbons.

Heptachlor is an organochlorine compound that was used as an insecticide. Usually sold as a white or tan powder, heptachlor is one of the cyclodiene insecticides. In 1962, Rachel Carson's Silent Spring questioned the safety of heptachlor and other chlorinated insecticides. Due to its highly stable structure, heptachlor can persist in the environment for decades. In the United States, the Environmental Protection Agency has limited the sale of heptachlor products to the specific application of fire ant control in underground transformers. The amount that can be present in different foods is regulated.
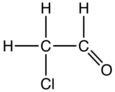
2-Chloroethanol (also called ethylene chlorohydrin or glycol chlorohydrin) is an organic chemical compound with the chemical formula HOCH2CH2Cl and the simplest beta-halohydrin (chlorohydrin). This colorless liquid has a pleasant ether-like odor. It is miscible with water. The molecule is bifunctional, consisting of both an alkyl chloride and an alcohol functional group.
1,2-Dichloroethylene or 1,2-DCE is the name for a pair of organochlorine compounds with the molecular formula C2H2Cl2. The two compounds are isomers, each being colorless liquids with a sweet odor. It can exist as either of two geometric isomers, cis-1,2-dichloroethene or trans-1,2-dichloroethene, but is often used as a mixture of the two. They have modest solubility in water. These compounds have some applications as a degreasing solvent. In contrast to most cis-trans compounds, the Z isomer (cis) is more stable than the E isomer (trans) by 0.4 kcal/mol.
1,1-Dichloroethylene, commonly called vinylidene chloride or 1,1-DCE, is an organochloride with the molecular formula CHCl2CH3. It is a colorless liquid with a sharp odor. Like most chlorocarbons, it is poorly soluble in water but soluble in organic solvents. 1,1-DCE was the precursor to the original clingwrap, Saran, for food, but this application has been phased out.
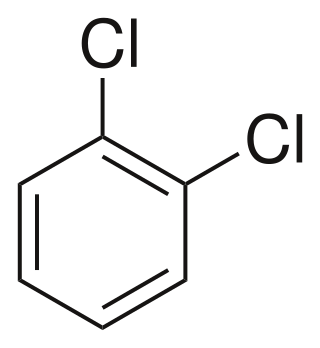
1,2-Dichlorobenzene, or orthodichlorobenzene (ODCB), is an aryl chloride and isomer of dichlorobenzene with the formula C6H4Cl2. This colourless liquid is poorly soluble in water but miscible with most organic solvents. It is a derivative of benzene, consisting of two adjacent chlorine atoms.
Perchloryl fluoride is a reactive gas with the chemical formula ClO
3F. It has a characteristic sweet odor that resembles gasoline and kerosene. It is toxic and is a powerful oxidizing and fluorinating agent. It is the acid fluoride of perchloric acid.

1,1,2,2-tetrachloroethane (TeCA), also known by the brand names Bonoform, Cellon and Westron, is an organic compound. It is colorless liquid and has a sweet odor. It is used as an industrial solvent and as a separation agent. TeCA is toxic and it can be inhaled, consumed or absorbed through the skin. After exposure, nausea, dizziness or even liver damage may occur.

Chloroacetyl chloride is a chlorinated acyl chloride. It is a bifunctional compound, making it a useful building block chemical.