
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sucrose and cholesterol. The presence of an OH group strongly modifies the properties of hydrocarbons, conferring hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur.
Acrylates are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion CH2=CHCO−2. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities.

Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually.

Ethyl acetate is the organic compound with the formula CH3CO2CH2CH3, simplified to C4H8O2. This colorless liquid has a characteristic sweet smell and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent.

Acetyl chloride is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.
In chemistry, solvolysis is a type of nucleophilic substitution (SN1/SN2) or elimination where the nucleophile is a solvent molecule. Characteristic of SN1 reactions, solvolysis of a chiral reactant affords the racemate. Sometimes however, the stereochemical course is complicated by intimate ion pairs, whereby the leaving anion remains close to the carbocation, effectively shielding it from an attack by the nucleophile. Particularly fast reactions can occur by neighbour group participation, with nonclassical ions as intermediates or transition states.
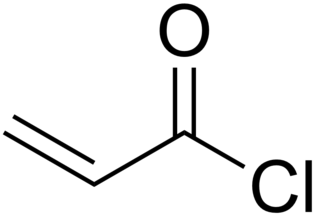
Acryloyl chloride, also known as 2-propenoyl chloride or acrylic acid chloride, is the organic compound with the formula CH2=CHCO(Cl). It is a colorless liquid, although aged samples appear yellow. It belongs to the acid chlorides group of compounds.

Terephthaloyl chloride is the acyl chloride of terephthalic acid. It is a white solid. It is one of two precursors used to make Kevlar, the other being p-phenylenediamine. TCL is used as a key component in performance polymers and aramid fibers, where it imparts flame resistance, chemical resistance, temperature stability, light weight, and very high strength. TCL is also an effective water scavenger, used to stabilize isocyanates and urethane prepolymers.
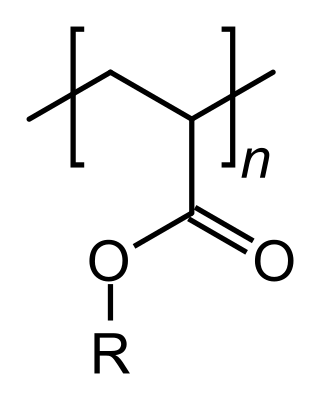
An acrylate polymer is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity.

Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)COOH. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).

Ethyl acrylate is an organic compound with the formula CH2CHCO2CH2CH3. It is the ethyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced for paints, textiles, and non-woven fibers. It is also a reagent in the synthesis of various pharmaceutical intermediates.

Methyl acrylate is an organic compound, more accurately the methyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced to make acrylate fiber, which is used to weave synthetic carpets. It is also a reagent in the synthesis of various pharmaceutical intermediates. Owing to the tendency of methyl acrylate to polymerize, samples typically contain an inhibitor such as hydroquinone.

Vinylsulfonic acid is the organosulfur compound with the chemical formula CH2=CHSO3H. It is the simplest unsaturated sulfonic acid. The C=C double bond is a site of high reactivity. Polymerization gives polyvinylsulfonic acid, especially when used as a comonomer with functionalized vinyl and (meth)acrylic acid compounds. It is a colorless, water-soluble liquid, although commercial samples can appear yellow or even red.
Butyl acrylate is an organic compound with the formula C4H9O2CCH=CH2. A colorless liquid, it is the butyl ester of acrylic acid. It is used commercially on a large scale as a precursor to poly(butyl acrylate). Especially as copolymers, such materials are used in paints, sealants, coatings, adhesives, fuel, textiles, plastics, and caulk.

N,N,N′,N′-Tetramethylformamidinium chloride is the simplest representative of quaternary formamidinium cations of the general formula [R2N−CH=NR2]+ with a chloride as a counterion in which all hydrogen atoms of the protonated formamidine [HC(=NH2)NH2]+ are replaced by methyl groups.

Tris(dimethylamino)methane (TDAM) is the simplest representative of the tris(dialkylamino)methanes of the general formula (R2N)3CH in which three of the four of methane's hydrogen atoms are replaced by dimethylamino groups (−N(CH3)2). Tris(dimethylamino)methane can be regarded as both an amine and an orthoamide.
1-Vinylimidazole is a water-soluble basic monomer that forms quaternizable homopolymers by free-radical polymerization with a variety of vinyl and acrylic monomers. The products are functional copolymers, which are used as oil field chemicals and as cosmetic auxiliaries. 1-Vinylimidazole acts as a reactive diluent in UV lacquers, inks, and adhesives.

3-Dimethylaminoacrolein is an organic compound with the formula Me2NC(H)=CHCHO. It is a pale yellow water-soluble liquid. The compound has a number of useful and unusual properties, e.g. it "causes a reversal of the hypnotic effect of morphine in mice" and has a "stimulating effect in humans".

N-(2-Carboxyethyl)iminodiacetic acid or β-ADA(β-alanine diacetate) is an organic compound with the formula HO2CCH2CH2N(CH2CO2H)2. It is a white solid. The compound is classified as an aminocarboxylic acid, formally a derivative of glycine.

α,β-Unsaturated carbonyl compounds are organic compounds with the general structure (O=CR)−Cα=Cβ-R. Such compounds include enones and enals, but also carboxylic acids and the corresponding esters and amides. In these compounds the carbonyl group is conjugated with an alkene. Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β-carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction.























