
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest halogen, and is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος ("stench"), referring to its sharp and pungent smell.

Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+
and hydroxide anions OH−
.

Boric acid, also called hydrogen borate, boracic acid, and orthoboric acid is a weak, monobasic Lewis acid of boron. However, some of its behaviour towards some chemical reactions suggest it to be tribasic acid in the Brønsted sense as well. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other chemical compounds. It has the chemical formula H3BO3 (sometimes written B(OH)3), and exists in the form of colorless crystals or a white powder that dissolves in water. When occurring as a mineral, it is called sassolite.
Borates are boron-oxygen compounds, which form boron oxyanions. These can be trigonal or tetrahedral in structure, or more loosely can consist of chemical mixtures which contain borate anions of either description. The element boron most often occurs in nature as borates, such as borate minerals and borosilicates.

Borax, also known as sodium borate decahydrate, is a white, granular mineral and sodium salt of boric acid.

Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, including being safely returned to the environment. Water treatment removes contaminants and undesirable components, or reduces their concentration so that the water becomes fit for its desired end-use. This treatment is crucial to human health and allows humans to benefit from both drinking and irrigation use.

Paint stripper, or paint remover, is a chemical product designed to remove paint, finishes, and coatings while also cleaning the underlying surface.

Tetrasodium pyrophosphate, also called sodium pyrophosphate, tetrasodium phosphate or TSPP, is an inorganic compound with the formula Na4P2O7. As a salt, it is a white, water-soluble solid. It is composed of pyrophosphate anion and sodium ions. Toxicity is approximately twice that of table salt when ingested orally. Also known is the decahydrate Na4P2O7 · 10(H2O).

Sodium oxalate, or disodium oxalate, is the sodium salt of oxalic acid with the formula Na2C2O4. It is a white, crystalline, odorless solid, that decomposes above 290 °C.
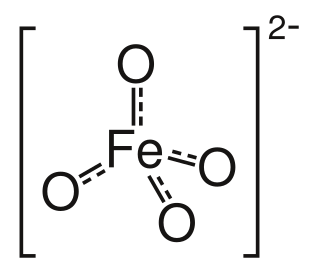
Ferrate(VI) is the inorganic anion with the chemical formula [FeO4]2−. It is photosensitive, contributes a pale violet colour to compounds and solutions containing it and is one of the strongest water-stable oxidizing species known. Although it is classified as a weak base, concentrated solutions containing ferrate(VI) are corrosive and attack the skin and are only stable at high pH.
Sodium perborate is chemical compound whose chemical formula may be written NaH
2BO
4, Na
2H
4B
2O
8, or, more properly, [Na+
]
2·[B
2O
4(OH)
4]2−
. Its name is sometimes abbreviated as PBS.

Calcium arsenate is the inorganic compound with the formula Ca3(AsO4)2. A colourless salt, it was originally used as a pesticide and as a germicide. It is highly soluble in water, as compared with lead arsenate, which makes it more toxic. The minerals rauenthalite Ca3(AsO4)2·10H2O and phaunouxite Ca3(AsO4)2·11H2O are hydrates of calcium arsenate.
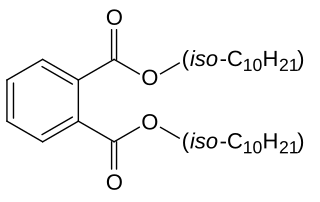
Diisodecyl phthalate (DIDP) is a commonly used plasticizer used in the production of plastic and plastic coating to increase flexibility. It is a mixture of compounds derived from the esterification of phthalic acid and isomeric decyl alcohols.
Hazard statements form part of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). They are intended to form a set of standardized phrases about the hazards of chemical substances and mixtures that can be translated into different languages. As such, they serve the same purpose as the well-known R-phrases, which they are intended to replace.

Trimethylborane (TMB) is a toxic, pyrophoric gas with the formula B(CH3)3 (which can also be written as Me3B, with Me representing methyl).

Calcium cyanide also known as black cyanide, is the calcium salt of hydrocyanic acid, an inorganic compound with the formula Ca(CN)2. The pure form is a white solid, although rarely observed; commercial samples can be black-gray. It hydrolyses readily (even in moist air) to release hydrogen cyanide. Like other similar cyanides it is very toxic.
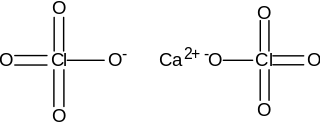
Calcium perchlorate is classified as a metal perchlorate salt with the molecular formula Ca(ClO4)2. It is an inorganic compound that is a yellow-white crystalline solid in appearance. As a strong oxidizing agent, it reacts with reducing agents when heated to generate heat and products that may be gaseous (which will cause pressurization in closed containers). Calcium perchlorate has been categorized as having explosive reactivity. Ca(ClO4)2 is a common chemical on the soil of planet Mars, counting for almost 1% of the Martian dust, by weight.
Magnesium chlorate is an inorganic chemical consisting of a magnesium cation and two chlorate anions: its chemical formula is Mg(ClO3)2.
Nitroxylic acid or hydronitrous acid is an unstable reduced oxonitrogen acid. It has formula H4N2O4 containing nitrogen in the +2 oxidation state. The corresponding anion called nitroxylate is N
2O4−
4 or NO2−
2.
Slimicide is a broad-spectrum antimicrobial pesticide used to kill slime-producing microorganisms such as algae, bacteria, fungi, and slime molds. One primary application domain is in the papermaking industry, where it reduces the occurrence of paper holes and spots, as well as protecting the machinery from odor, clogs, corrosion, and breakdown. Slimicides come in variants effective in acidic and/or alkaline media, in liquid or solid form, and are based on chemicals such as aldehydes, bromium or quaternary ammonium compounds, and others. Additional significant application areas for slimicides include industrial water recirculation systems such as cooling towers, fuel storage tanks and wells, and in conjunction with fluids used for oil extraction. In some application domains, slimicides may be formulated specifically to target slime molds.













