Related Research Articles
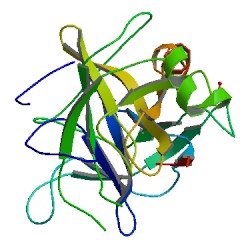
Chymotrypsin (EC 3.4.21.1, chymotrypsins A and B, alpha-chymar ophth, avazyme, chymar, chymotest, enzeon, quimar, quimotrase, alpha-chymar, alpha-chymotrypsin A, alpha-chymotrypsin) is a digestive enzyme component of pancreatic juice acting in the duodenum, where it performs proteolysis, the breakdown of proteins and polypeptides. Chymotrypsin preferentially cleaves peptide amide bonds where the side chain of the amino acid N-terminal to the scissile amide bond (the P1 position) is a large hydrophobic amino acid (tyrosine, tryptophan, and phenylalanine). These amino acids contain an aromatic ring in their side chain that fits into a hydrophobic pocket (the S1 position) of the enzyme. It is activated in the presence of trypsin. The hydrophobic and shape complementarity between the peptide substrate P1 side chain and the enzyme S1 binding cavity accounts for the substrate specificity of this enzyme. Chymotrypsin also hydrolyzes other amide bonds in peptides at slower rates, particularly those containing leucine at the P1 position.

Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsinogen proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized. Trypsin was discovered in 1876 by Wilhelm Kühne and was named from the Ancient Greek word for rubbing since it was first isolated by rubbing the pancreas with glycerin.

A protease is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in numerous biological pathways, including digestion of ingested proteins, protein catabolism, and cell signaling.

In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the binding site, and residues that catalyse a reaction of that substrate, the catalytic site. Although the active site occupies only ~10–20% of the volume of an enzyme, it is the most important part as it directly catalyzes the chemical reaction. It usually consists of three to four amino acids, while other amino acids within the protein are required to maintain the tertiary structure of the enzymes.
In biology and biochemistry, protease inhibitors, or antiproteases, are molecules that inhibit the function of proteases. Many naturally occurring protease inhibitors are proteins.
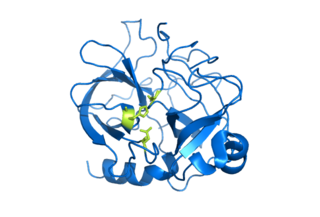
Serine proteases are enzymes that cleave peptide bonds in proteins. Serine serves as the nucleophilic amino acid at the (enzyme's) active site. They are found ubiquitously in both eukaryotes and prokaryotes. Serine proteases fall into two broad categories based on their structure: chymotrypsin-like (trypsin-like) or subtilisin-like.

A metalloproteinase, or metalloprotease, is any protease enzyme whose catalytic mechanism involves a metal. An example is ADAM12 which plays a significant role in the fusion of muscle cells during embryo development, in a process known as myogenesis.
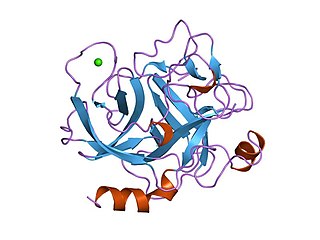
Endopeptidase or endoproteinase are proteolytic peptidases that break peptide bonds of nonterminal amino acids, in contrast to exopeptidases, which break peptide bonds from end-pieces of terminal amino acids. For this reason, endopeptidases cannot break down peptides into monomers, while exopeptidases can break down proteins into monomers. A particular case of endopeptidase is the oligopeptidase, whose substrates are oligopeptides instead of proteins.
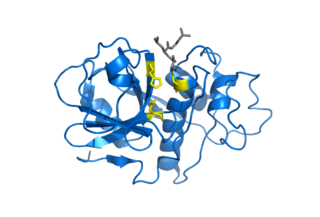
Cysteine proteases, also known as thiol proteases, are hydrolase enzymes that degrade proteins. These proteases share a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic triad or dyad.
Amprenavir is a protease inhibitor used to treat HIV infection. It was approved by the Food and Drug Administration on April 15, 1999, for twice-a-day dosing instead of needing to be taken every eight hours. The convenient dosing came at a price, as the dose required is 1,200 mg, delivered in 8 (eight) very large 150 mg gel capsules or 24 (twenty-four) 50 mg gel capsules twice daily.
Protein metabolism denotes the various biochemical processes responsible for the synthesis of proteins and amino acids (anabolism), and the breakdown of proteins by catabolism.

Aspartic proteases are a catalytic type of protease enzymes that use an activated water molecule bound to one or more aspartate residues for catalysis of their peptide substrates. In general, they have two highly conserved aspartates in the active site and are optimally active at acidic pH. Nearly all known aspartyl proteases are inhibited by pepstatin.
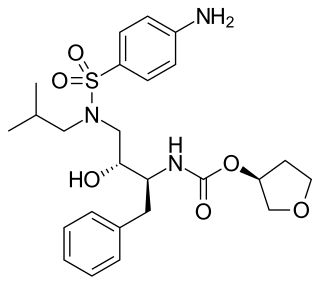
Omapatrilat is an experimental antihypertensive agent that was never marketed. It inhibits both neprilysin and angiotensin-converting enzyme (ACE). NEP inhibition results in elevated natriuretic peptide levels, promoting natriuresis, diuresis, vasodilation, and reductions in preload and ventricular remodeling.

Ecadotril is a neutral endopeptidase inhibitor ((NEP) EC 3.4.24.11) and determined by the presence of peptidase family M13 as a neutral endopeptidase inhibited by phosphoramidon. Ecadotril is the (S)-enantiomer of racecadotril. NEP-like enzymes include the endothelin-converting enzymes. The peptidase M13 family believed to activate or inactivate oligopeptide (pro)-hormones such as opioid peptides, neprilysin is another member of this group, in the case of the metallopeptidases and aspartic, the nucleophiles clan or family for example MA, is an activated water molecule. The peptidase domain for members of this family also contains a bacterial member and resembles that of thermolysin the predicted active site residues for members of this family and thermolysin occur in the motif HEXXH. Thermolysin complexed with the inhibitor (S)-thiorphan are isomeric thiol-containing inhibitors of endopeptidase EC 24-11 (also called "enkephalinase").

Candoxatril is the orally active prodrug of candoxatrilat (UK-73967).

An Oligopeptidase is an enzyme that cleaves peptides but not proteins. This property is due to its structure: the active site of this enzyme is located at the end of a narrow cavity which can only be reached by peptides.
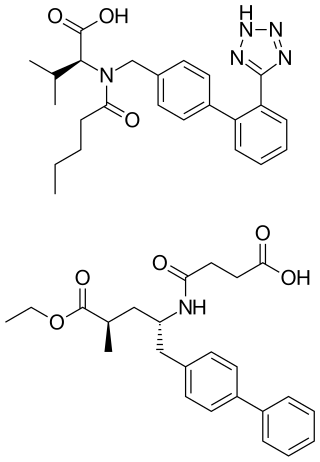
Sacubitril/valsartan, sold under the brand name Entresto, is a fixed-dose combination medication for use in heart failure. It consists of the neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan. The combination is sometimes described as an "angiotensin receptor-neprilysin inhibitor" (ARNi). In 2016, the American College of Cardiology/American Heart Association Task Force recommended it as a replacement for an ACE inhibitor or an angiotensin receptor blocker in people with heart failure with reduced ejection fraction.
Alpha-lytic endopeptidase or Alpha-lytic protease is an enzyme isolated from the myxobacterium Lysobacter enzymogenes. This enzyme is a serine protease that catalyses the breakage of peptide bonds using a hydrolysis chemical reaction. Alpha-lytic protease was named based on the observed cleavage specificity for the α position of the tetrapeptide component in gram-positive bacterial cell walls (alanine). Alpha-lytic protease is also capable of digesting elastin and other proteins.

The PA clan is the largest group of proteases with common ancestry as identified by structural homology. Members have a chymotrypsin-like fold and similar proteolysis mechanisms but can have identity of <10%. The clan contains both cysteine and serine proteases. PA clan proteases can be found in plants, animals, fungi, eubacteria, archaea and viruses.
An exopeptidase inhibitor is a drug that inhibits one or more exopeptidase enzymes. Exopeptidases are one of two types of proteases, the other being endopeptidases. Exopeptidases cleave peptide bonds of terminal amino acids, resulting in the release of a single amino acid or dipeptide from the peptide chain, whereas endoeptidases break non-terminal bonds.