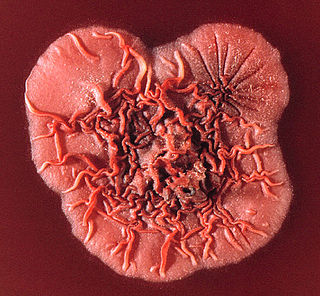
Talaromyces marneffei, formerly called Penicillium marneffei, was identified in 1956. The organism is endemic to southeast Asia, where it is an important cause of opportunistic infections in those with HIV/AIDS-related immunodeficiency. Incidence of T. marneffei infections has increased due to a rise in HIV infection rates in the region.

Fungal infection, also known as mycosis, is a disease caused by fungi. Different types are traditionally divided according to the part of the body affected; superficial, subcutaneous, and systemic. Superficial fungal infections include common tinea of the skin, such as tinea of the body, groin, hands, feet and beard, and yeast infections such as pityriasis versicolor. Subcutaneous types include eumycetoma and chromoblastomycosis, which generally affect tissues in and beneath the skin. Systemic fungal infections are more serious and include cryptococcosis, histoplasmosis, pneumocystis pneumonia, aspergillosis and mucormycosis. Signs and symptoms range widely. There is usually a rash with superficial infection. Fungal infection within the skin or under the skin may present with a lump and skin changes. Pneumonia-like symptoms or meningitis may occur with a deeper or systemic infection.

Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.
Exophiala jeanselmei is a saprotrophic fungus in the family Herpotrichiellaceae. Four varieties have been discovered: Exophiala jeanselmei var. heteromorpha, E. jeanselmei var. lecanii-corni, E. jeanselmei var. jeanselmei, and E. jeanselmei var. castellanii. Other species in the genus Exophiala such as E. dermatitidis and E. spinifera have been reported to have similar annellidic conidiogenesis and may therefore be difficult to differentiate.

Blastomyces dermatitidis is a dimorphic fungus that causes blastomycosis, an invasive and often serious fungal infection found occasionally in humans and other animals. It lives in soil and wet, decaying wood, often in an area close to a waterway such as a lake, river or stream. Indoor growth may also occur, for example, in accumulated debris in damp sheds or shacks. The fungus is endemic to parts of eastern North America, particularly boreal northern Ontario, southeastern Manitoba, Quebec south of the St. Lawrence River, parts of the U.S. Appalachian mountains and interconnected eastern mountain chains, the west bank of Lake Michigan, the state of Wisconsin, and the entire Mississippi Valley including the valleys of some major tributaries such as the Ohio River. In addition, it occurs rarely in Africa both north and south of the Sahara Desert, as well as in the Arabian Peninsula and the Indian subcontinent. Though it has never been directly observed growing in nature, it is thought to grow there as a cottony white mold, similar to the growth seen in artificial culture at 25 °C (77 °F). In an infected human or animal, however, it converts in growth form and becomes a large-celled budding yeast. Blastomycosis is generally readily treatable with systemic antifungal drugs once it is correctly diagnosed; however, delayed diagnosis is very common except in highly endemic areas.

Microbial corneal infection is the most serious and "most common vision threatening" complication of contact lens wear, which is believed to be strongly associated with contact lens cases. Such infections "are being increasingly recognized as an important cause of morbidity and blindness" and "may even be life-threatening." While the cornea is believed to be the most common site for fungal eye infections, other parts of the eye such as the orbit, sclera, eyelids, and more may also be involved. Contact lens cases are recognized as a "potential source of pathogens associated with corneal ulcers" and according to Moorfields Eye Hospital, contact lens wear is “the most prevalent risk factor for new cases of corneal ulcers.” Contaminants "isolated from contact lens associated corneal ulcers have often been shown to be" the same as found in the patient's contact lens case, thus providing evidence contaminated contact lens cases may be a "replenishable source of pathogenic microbes."
Black yeasts, sometimes also black fungi, dematiaceous fungi, microcolonial fungi or meristematic fungi is a diverse group of slow-growing microfungi which reproduce mostly asexually. Only few genera reproduce by budding cells, while in others hyphal or meristematic (isodiametric) reproduction is preponderant. Black yeasts share some distinctive characteristics, in particular a dark colouration (melanisation) of their cell wall. Morphological plasticity, incrustation of the cell wall with melanins and presence of other protective substances like carotenoids and mycosporines represent passive physiological adaptations which enable black fungi to be highly resistant against environmental stresses. The term "polyextremotolerance" has been introduced to describe this phenotype, an example of which is the species Aureobasidium pullulans. Presence of 1,8-dihydroxynaphthalene melanin in the cell wall confers to the microfungi their characteristic olivaceous to dark brown/black colour.
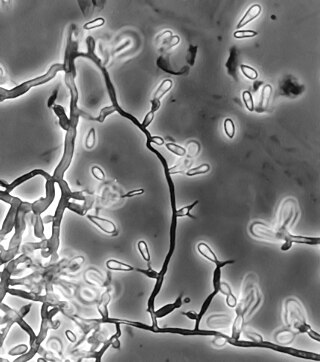
Ochroconis gallopava, also called Dactylaria gallopava or Dactylaria constricta var. gallopava, is a member of genus Dactylaria. Ochroconis gallopava is a thermotolerant, darkly pigmented fungus that causes various infections in fowls, turkeys, poults, and immunocompromised humans first reported in 1986. Since then, the fungus has been increasingly reported as an agent of human disease especially in recipients of solid organ transplants. Ochroconis gallopava infection has a long onset and can involve a variety of body sites. Treatment of infection often involves a combination of antifungal drug therapy and surgical excision.

Cladophialophora bantiana is a melanin producing mold known to cause brain abscesses in humans. It is one of the most common causes of systemic phaeohyphomycosis in mammals. Cladophialophora bantiana is a member of the ascomycota and has been isolated from soil samples from around the world.
Phaeohyphomycosis is a diverse group of fungal infections, caused by dematiaceous fungi whose morphologic characteristics in tissue include hyphae, yeast-like cells, or a combination of these. It can be associated with an array of melanistic filamentous fungi including Alternaria species, Exophiala jeanselmei, and Rhinocladiella mackenziei.
Scedosporiosis is the general name for any mycosis – i.e., fungal infection – caused by a fungus from the genus Scedosporium. Current population-based studies suggest Scedosporium prolificans and Scedosporium apiospermum to be among the most common infecting agents from the genus, although infections caused by other members thereof are not unheard of. The latter is an asexual form (anamorph) of another fungus, Pseudallescheria boydii. The former is a "black yeast", currently not characterized as well, although both of them have been described as saprophytes.

Exophiala phaeomuriformis is thermophilic fungus belonging to the genus Exophiala and the family Herpotrichiellaceae. it is a member of the group of fungi known as black yeasts, and is typically found in hot and humid locations, such as saunas, bathrooms, and dishwashers. This species can cause skin infections and is typically classified as a Biosafety Risk Group 2 agent.
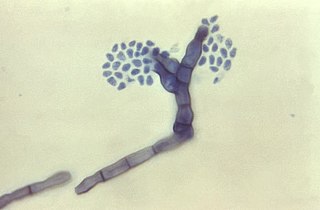
Fonsecaea compacta is a saprophytic fungal species found in the family Herpotrichiellaceae. It is a rare etiological agent of chromoblastomycosis, with low rates of correspondence observed from reports. The main active components of F. compacta are glycolipids, yet very little is known about its composition. F. compacta is widely regarded as a dysplastic variety of Fonsecaea pedrosoi, its morphological precursor. The genus Fonsecaea presently contains two species, F. pedrosoi and F. compacta. Over 100 strains of F. pedrosoi have been isolated but only two of F. compacta.

Rhinocladiella mackenziei is a deeply pigmented mold that is a common cause of human cerebral phaeohyphomycosis. Rhinocladiella mackenziei was believed to be endemic solely to the Middle East, due to the first cases of infection being limited to the region. However, cases of R. mackenziei infection are increasingly reported from regions outside the Middle East. This pathogen is unique in that the majority of cases have been reported from immunologically normal people.

Phialophora verrucosa is a pathogenic, dematiaceous fungus that is a common cause of chromoblastomycosis. It has also been reported to cause subcutaneous phaeohyphomycosis and mycetoma in very rare cases. In the natural environment, it can be found in rotting wood, soil, wasp nests, and plant debris. P. verrucosa is sometimes referred to as Phialophora americana, a closely related environmental species which, along with P. verrucosa, is also categorized in the P. carrionii clade.
Cladosporium oxysporum is an airborne fungus that is commonly found outdoors and is distributed throughout the tropical and subtropical region, it is mostly located In Asia and Africa. It spreads through airborne spores and is often extremely abundant in outdoor air during the spring and summer seasons. It mainly feeds on decomposing organic matter in warmer climates, but can also be parasitic and feed on living plants. The airborne spores can occasionally cause cutaneous infections in humans, and the high prevalence of C. oxysporum in outdoor air during warm seasons contributes to its importance as an etiological agent of allergic disease and possibly human cutaneous phaeohyphomycosis in tropical regions.
Exophiala pisciphila is a mesophilic black yeast and member of the dark septate endophytes. This saprotrophic fungus is found commonly in marine and soil environments. It is abundant in harsh environments like soil contaminated with heavy metals. E. pisciphila forms symbiotic relationships with various plants by colonizing on roots, conferring resistance to drought and heavy metal stress. It is an opportunistic pathogen that commonly causes infections in captive fish and amphibians, while rarely causing disease in humans. Secondary metabolites produced by this species have potential clinical antibiotic and antiretroviral applications.

Trichosporon asteroides is an asexual basidiomycetous fungus first described from human skin but now mainly isolated from blood and urine. T. asteroides is a hyphal fungus with a characteristically yeast-like appearance due to the presence of slimy arthroconidia. Infections by this species usually respond to treatment with azoles and amphotericin B.
Arthrographis kalrae is an ascomycetous fungus responsible for human nail infections described in 1938 by Cochet as A. langeronii. A. kalrae is considered a weak pathogen of animals including human restricted to the outermost keratinized layers of tissue. Infections caused by this species are normally responsive to commonly used antifungal drugs with only very rare exceptions.
Cladophialophora arxii is a black yeast shaped dematiaceous fungus that is able to cause serious phaeohyphomycotic infections. C. arxii was first discovered in 1995 in Germany from a 22-year-old female patient suffering multiple granulomatous tracheal tumours. It is a clinical strain that is typically found in humans and is also capable of acting as an opportunistic fungus of other vertebrates Human cases caused by C. arxii have been reported from all parts of the world such as Germany and Australia.












