In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a molecule. Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic field. This re-orientation occurs with absorption of electromagnetic radiation in the radio frequency region from roughly 4 to 900 MHz, which depends on the isotopic nature of the nucleus and increased proportionally to the strength of the external magnetic field. Notably, the resonance frequency of each NMR-active nucleus depends on its chemical environment. As a result, NMR spectra provide information about individual functional groups present in the sample, as well as about connections between nearby nuclei in the same molecule. As the NMR spectra are unique or highly characteristic to individual compounds and functional groups, NMR spectroscopy is one of the most important methods to identify molecular structures, particularly of organic compounds.

Solid-state NMR (ssNMR) spectroscopy is a technique for characterizing atomic level structure in solid materials e.g. powders, single crystals and amorphous samples and tissues using nuclear magnetic resonance (NMR) spectroscopy. The anisotropic part of many spin interactions are present in solid-state NMR, unlike in solution-state NMR where rapid tumbling motion averages out many of the spin interactions. As a result, solid-state NMR spectra are characterised by larger linewidths than in solution state NMR, which can be utilized to give quantitative information on the molecular structure, conformation and dynamics of the material. Solid-state NMR is often combined with magic angle spinning to remove anisotropic interactions and improve the resolution as well as the sensitivity of the technique.
Two-dimensional nuclear magnetic resonance spectroscopy is a set of nuclear magnetic resonance spectroscopy (NMR) methods which give data plotted in a space defined by two frequency axes rather than one. Types of 2D NMR include correlation spectroscopy (COSY), J-spectroscopy, exchange spectroscopy (EXSY), and nuclear Overhauser effect spectroscopy (NOESY). Two-dimensional NMR spectra provide more information about a molecule than one-dimensional NMR spectra and are especially useful in determining the structure of a molecule, particularly for molecules that are too complicated to work with using one-dimensional NMR.

Alexander Pines is an American chemist. He is the Glenn T. Seaborg Professor Emeritus, University of California, Berkeley, Chancellor's Professor Emeritus and Professor of the Graduate School, University of California, Berkeley, and a member of the California Institute for Quantitative Biosciences (QB3) and the Department of Bioengineering. He was born in 1945, grew up in Bulawayo in Southern Rhodesia and studied undergraduate mathematics and chemistry in Israel at Hebrew University of Jerusalem. Coming to the United States in 1968, Pines obtained his Ph.D. in chemical physics at M.I.T. in 1972 and joined the UC Berkeley faculty later that year.
Insensitive nuclei enhancement by polarization transfer (INEPT) is a signal enhancement method used in NMR spectroscopy. It involves the transfer of nuclear spin polarization from spins with large Boltzmann population differences to nuclear spins of interest with lower Boltzmann population differences. INEPT uses J-coupling for the polarization transfer in contrast to Nuclear Overhauser effect (NOE), which arises from dipolar cross-relaxation. This method of signal enhancement was introduced by Ray Freeman in 1979. Due to its usefulness in signal enhancement, pulse sequences used in heteronuclear NMR experiments often contain blocks of INEPT or INEPT-like sequences.
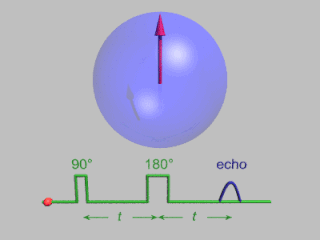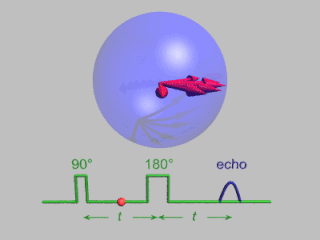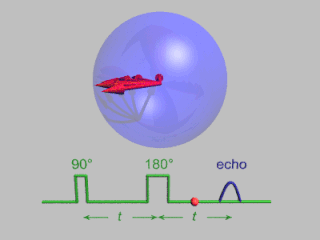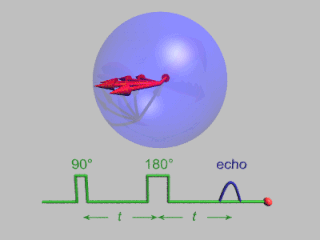
In magnetic resonance, a spin echo or Hahn echo is the refocusing of spin magnetisation by a pulse of resonant electromagnetic radiation. Modern nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI) make use of this effect.
A pulsed field gradient is a short, timed pulse with spatial-dependent field intensity. Any gradient is identified by four characteristics: axis, strength, shape and duration.
Fast low angle shot magnetic resonance imaging is a particular sequence of magnetic resonance imaging. It is a gradient echo sequence which combines a low-flip angle radio-frequency excitation of the nuclear magnetic resonance signal with a short repetition time. It is the generic form of steady-state free precession imaging.

Zero- to ultralow-field (ZULF) NMR is the acquisition of nuclear magnetic resonance (NMR) spectra of chemicals with magnetically active nuclei in an environment carefully screened from magnetic fields. ZULF NMR experiments typically involve the use of passive or active shielding to attenuate Earth’s magnetic field. This is in contrast to the majority of NMR experiments which are performed in high magnetic fields provided by superconducting magnets. In ZULF experiments the sample is moved through a low field magnet into the "zero field" region where the dominant interactions are nuclear spin-spin couplings, and the coupling between spins and the external magnetic field is a perturbation to this. There are a number of advantages to operating in this regime: magnetic-susceptibility-induced line broadening is attenuated which reduces inhomogeneous broadening of the spectral lines for samples in heterogeneous environments. Another advantage is that the low frequency signals readily pass through conductive materials such as metals due to the increased skin depth; this is not the case for high-field NMR for which the sample containers are usually made of glass, quartz or ceramic. High-field NMR employs inductive detectors to pick up the radiofrequency signals, but this would be inefficient in ZULF NMR experiments since the signal frequencies are typically much lower. The development of highly sensitive magnetic sensors in the early 2000s including SQUIDs, magnetoresistive sensors, and SERF atomic magnetometers made it possible to detect NMR signals directly in the ZULF regime. Previous ZULF NMR experiments relied on indirect detection where the sample had to be shuttled from the shielded ZULF environment into a high magnetic field for detection with a conventional inductive pick-up coil. One successful implementation was using atomic magnetometers at zero magnetic field working with rubidium vapor cells to detect zero-field NMR.
Herbert Sander Gutowsky was an American chemist who was a professor of chemistry at the University of Illinois Urbana-Champaign. Gutowsky was the first to apply nuclear magnetic resonance (NMR) methods to the field of chemistry. He used nuclear magnetic resonance spectroscopy to determine the structure of molecules. His pioneering work developed experimental control of NMR as a scientific instrument, connected experimental observations with theoretical models, and made NMR one of the most effective analytical tools for analysis of molecular structure and dynamics in liquids, solids, and gases, used in chemical and medical research, His work was relevant to the solving of problems in chemistry, biochemistry, and materials science, and has influenced many of the subfields of more recent NMR spectroscopy.
In vivo magnetic resonance spectroscopy (MRS) is a specialized technique associated with magnetic resonance imaging (MRI).
Raymond Freeman FRS was a British chemist and professor at Jesus College, Cambridge who made important contributions to NMR spectroscopy.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. High-resolution nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI). The original application of NMR to condensed matter physics is nowadays mostly devoted to strongly correlated electron systems. It reveals large many-body couplings by fast broadband detection and should not be confused with solid state NMR, which aims at removing the effect of the same couplings by Magic Angle Spinning techniques.
Triple resonance experiments are a set of multi-dimensional nuclear magnetic resonance spectroscopy (NMR) experiments that link three types of atomic nuclei, most typically consisting of 1H, 15N and 13C. These experiments are often used to assign specific resonance signals to specific atoms in an isotopically-enriched protein. The technique was first described in papers by Ad Bax, Mitsuhiko Ikura and Lewis Kay in 1990, and further experiments were then added to the suite of experiments. Many of these experiments have since become the standard set of experiments used for sequential assignment of NMR resonances in the determination of protein structure by NMR. They are now an integral part of solution NMR study of proteins, and they may also be used in solid-state NMR.
Nitrogen-15 nuclear magnetic resonance spectroscopy is a version of nuclear magnetic resonance spectroscopy that examines samples containing the 15N nucleus. 15N NMR differs in several ways from the more common 13C and 1H NMR. To circumvent the difficulties associated with measurement of the quadrupolar, spin-1 14N nuclide, 15N NMR is employed in samples for detection since it has a ground-state spin of ½. Since14N is 99.64% abundant, incorporation of 15N into samples often requires novel synthetic techniques.

Geoffrey Bodenhausen is a French chemist specializing in nuclear magnetic resonance, being highly cited in his field. He is a Corresponding member of the Royal Netherlands Academy of Arts and Sciences and a Fellow of the American Physical Society. He is professeur émérite at the Department of Chemistry at the École Normale Supérieure (ENS) in Paris and professeur honoraire at the Laboratory of Biomolecular Magnetic Resonance of the École Polytechnique Fédérale de Lausanne (EPFL). He is a member of the editorial board of the journal Progress in Nuclear Magnetic Resonance Spectroscopy. He is the chair of the editorial board of the journal Magnetic Resonance.
The Russell Varian Prize was an international scientific prize awarded for a single, high-impact and innovative contribution in the field of nuclear magnetic resonance (NMR), that laid the foundation for the development of new technologies in the field. It honored the memory of Russell Varian, the pioneer behind the creation of the first commercial NMR spectrometer and the co-founder, in 1948, of Varian Associates, one of the first high-tech companies in Silicon Valley. The prize carried a monetary award of €15,000 and it was awarded annually between the years 2002 and 2015 by a committee of experts in the field. The award ceremony alternated between the European Magnetic Resonance (EUROMAR) Conference and the International Council on Magnetic Resonance in Biological Systems (ICMRBS) Conference. Originally, the prize was sponsored by Varian, Inc. and later by Agilent Technologies, after the latter acquired Varian, Inc. in 2010. The prize was discontinued in 2016 after Agilent Technologies closed its NMR division.
Malcolm Harris Levitt is a British physical chemist and nuclear magnetic resonance (NMR) spectroscopist. He is Professor in Physical Chemistry at the University of Southampton and was elected a Fellow of the Royal Society in 2007.

Alfred G. Redfield was an American physicist and biochemist. In 1955 he published the Redfield relaxation theory, effectively moving the practice of NMR or Nuclear magnetic resonance from the realm of classical physics to the realm of semiclassical physics. He continued to find novel magnetic resonance applications to solve real-world problems throughout his life.








