
In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can change into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage. They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type.

Bone marrow is a semi-solid tissue found within the spongy portions of bones. In birds and mammals, bone marrow is the primary site of new blood cell production. It is composed of hematopoietic cells, marrow adipose tissue, and supportive stromal cells. In adult humans, bone marrow is primarily located in the ribs, vertebrae, sternum, and bones of the pelvis. Bone marrow comprises approximately 5% of total body mass in healthy adult humans, such that a man weighing 73 kg (161 lbs) will have around 3.7 kg (8 lbs) of bone marrow.

Hematopoietic stem cells (HSCs) are the stem cells that give rise to other blood cells. This process is called haematopoiesis. In vertebrates, the first definitive HSCs arise from the ventral endothelial wall of the embryonic aorta within the (midgestational) aorta-gonad-mesonephros region, through a process known as endothelial-to-hematopoietic transition. In adults, haematopoiesis occurs in the red bone marrow, in the core of most bones. The red bone marrow is derived from the layer of the embryo called the mesoderm.

CD34 is a transmembrane phosphoglycoprotein protein encoded by the CD34 gene in humans, mice, rats and other species.

Ernest Armstrong McCulloch was a University of Toronto cellular biologist, best known for demonstrating – with James Till – the existence of stem cells.
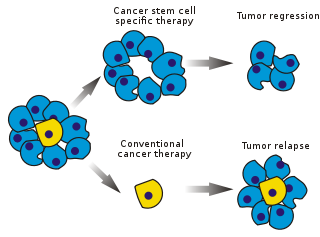
Cancer stem cells (CSCs) are cancer cells that possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample. CSCs are therefore tumorigenic (tumor-forming), perhaps in contrast to other non-tumorigenic cancer cells. CSCs may generate tumors through the stem cell processes of self-renewal and differentiation into multiple cell types. Such cells are hypothesized to persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. Therefore, development of specific therapies targeted at CSCs holds hope for improvement of survival and quality of life of cancer patients, especially for patients with metastatic disease.
Tak Wah Mak, is a Canadian medical researcher, geneticist, oncologist, and biochemist. He first became widely known for his discovery of the T-cell receptor in 1983 and pioneering work in the genetics of immunology. In 1995, Mak published a landmark paper on the discovery of the function of the immune checkpoint protein CTLA-4, thus opening the path for immunotherapy/checkpoint inhibitors as a means of cancer treatment. Mak is also the founder of Agios Pharmaceuticals, whose lead compound, IDHIFA®, was approved by the FDA for acute myeloid leukemia in August 2017, becoming the first drug specifically targeting cancer metabolism to be used for cancer treatment. He has worked in a variety of areas including biochemistry, immunology, and cancer genetics.

Adult stem cells are undifferentiated cells, found throughout the body after development, that multiply by cell division to replenish dying cells and regenerate damaged tissues. Also known as somatic stem cells, they can be found in juvenile, adult animals, and humans, unlike embryonic stem cells.

X-linked severe combined immunodeficiency (X-SCID) is an immunodeficiency disorder in which the body produces very few T cells and NK cells.

Wilms tumor protein (WT33) is a protein that in humans is encoded by the WT1 gene on chromosome 11p.

Homeobox protein Hox-A9 is a protein that in humans is encoded by the HOXA9 gene.

Sean J. Morrison is a Canadian-American stem cell biologist and cancer researcher. Morrison is the director of Children's Medical Center Research Institute at UT Southwestern (CRI), a nonprofit research institute established in 2011 as a joint venture between Children’s Health System of Texas and UT Southwestern Medical Center. With Morrison as founding director, CRI was established to perform transformative biomedical research at the interface of stem cell biology, cancer and metabolism to better understand the biological basis of disease. He is a Howard Hughes Medical Institute Investigator, has served as president of the International Society for Stem Cell Research, and is a member of the U.S. National Academy of Medicine, U.S. National Academy of Sciences and European Molecular Biology Organization.
A humanized mouse is a genetically modified mouse that has functioning human genes, cells, tissues and/or organs. Humanized mice are commonly used as small animal models in biological and medical research for human therapeutics.
The NSG mouse is a brand of immunodeficient laboratory mice, developed and marketed by Jackson Laboratory, which carries the strain NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ. NSG branded mice are among the most immunodeficient described to date. NSG branded mice lack mature T cells, B cells, and natural killer (NK) cells. NSG branded mice are also deficient in multiple cytokine signaling pathways, and they have many defects in innate immunity. The compound immunodeficiencies in NSG branded mice permit the engraftment of a wide range of primary human cells, and enable sophisticated modeling of many areas of human biology and disease. NSG branded mice were developed in the laboratory of Dr. Leonard Shultz at Jackson Laboratory, which owns the NSG trade mark.
Allen Charles Edward Eaves is the co-founding Director of the Terry Fox Laboratory for Hematology/Oncology Research, which over a 25-year period (1981–2006) he grew into an internationally recognized centre for the study of leukemia and stem cell research. His own research on chronic myelogenous leukemia (CML) has led the way to a new understanding of the disease. As Head of Hematology at the British Columbia Cancer Agency and the University of British Columbia for 18 years (1985–2003) he engineered the building of one of the first and largest bone marrow transplant programs in Canada. In recognition of his research accomplishments and leadership in moving basic science discoveries in stem cell biology into the clinic, he was elected President of the International Society of Cellular Therapy (1995–1997), Treasurer of the Foundation for the Accreditation of Cellular Therapy (1995–2002) and President of the American Society of Blood and Marrow Transplantation (1999–2000). In 2003 he was awarded the prestigious R. M. Taylor Medal by the Canadian Cancer Society and the National Cancer Institute of Canada.
Many human blood cells, such as red blood cells (RBCs), immune cells, and even platelets all originate from the same progenitor cell, the hematopoietic stem cell (HSC). As these cells are short-lived, there needs to be a steady turnover of new blood cells and the maintenance of an HSC pool. This is broadly termed hematopoiesis. This event requires a special environment, termed the hematopoietic stem cell niche, which provides the protection and signals necessary to carry out the differentiation of cells from HSC progenitors. This stem-cell niche relocates from the yolk sac to eventually rest in the bone marrow of mammals. Many pathological states can arise from disturbances in this niche environment, highlighting its importance in maintaining hematopoiesis.
Patient derived xenografts (PDX) are models of cancer where the tissue or cells from a patient's tumor are implanted into an immunodeficient or humanized mouse. It is a form of xenotransplantation. PDX models are used to create an environment that allows for the continued growth of cancer after its removal from a patient. In this way, tumor growth can be monitored in the laboratory, including in response to potential therapeutic options. Cohorts of PDX models can be used to determine the therapeutic efficiency of a therapy against particular types of cancer, or a PDX model from a specific patient can be tested against a range of therapies in a 'personalized oncology' approach.
Mice with severe combined immunodeficiency (SCIDs) are often used in the research of human disease. Human immune cells are used to develop human lymphoid organs within these immunodeficient mice, and many different types of SCID mouse models have been developed. These mice allow researchers to study the human immune system and human disease in a small animal model.

Shimon Slavin is an Israeli professor of medicine. He pioneered immunotherapy mediated by allogeneic donor lymphocytes and innovative methods for stem cell transplantation to cure hematological malignancies and solid tumors. He also used hematopoietic stem cells to induce transplantation tolerance to bone marrow and organ allografts.
Since haematopoietic stem cells cannot be isolated as a pure population, it is not possible to identify them under a microscope. Therefore, there are many techniques to isolate haematopoietic stem cells (HSCs). HSCs can be identified or isolated by the use of flow cytometry where the combination of several different cell surface markers is used to separate the rare HSCs from the surrounding blood cells. HSCs lack expression of mature blood cell markers and are thus, called Lin-. Lack of expression of lineage markers is used in combination with detection of several positive cell-surface markers to isolate HSCs. In addition, HSCs are characterized by their small size and low staining with vital dyes such as rhodamine 123 or Hoechst 33342.











