
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are a class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from virucides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural virucides are produced by some plants such as eucalyptus and Australian tea trees.

Antigenic shift is the process by which two or more different strains of a virus, or strains of two or more different viruses, combine to form a new subtype having a mixture of the surface antigens of the two or more original strains. The term is often applied specifically to influenza, as that is the best-known example, but the process is also known to occur with other viruses, such as visna virus in sheep. Antigenic shift is a specific case of reassortment or viral shift that confers a phenotypic change.

Influenza hemagglutinin (HA) or haemagglutinin[p] is a homotrimeric glycoprotein found on the surface of influenza viruses and is integral to its infectivity.
Antigenic drift is a kind of genetic variation in viruses, arising from the accumulation of mutations in the virus genes that code for virus-surface proteins that host antibodies recognize. This results in a new strain of virus particles that is not effectively inhibited by the antibodies that prevented infection by previous strains. This makes it easier for the changed virus to spread throughout a partially immune population. Antigenic drift occurs in both influenza A and influenza B viruses.

Zanamivir is a medication used to treat and prevent influenza caused by influenza A and influenza B viruses. It is a neuraminidase inhibitor and was developed by the Australian biotech firm Biota Holdings. It was licensed to Glaxo in 1990 and approved in the US in 1999, only for use as a treatment for influenza. In 2006, it was approved for prevention of influenza A and B. Zanamivir was the first neuraminidase inhibitor commercially developed. It is marketed by GlaxoSmithKline under the trade name Relenza as a powder for oral inhalation.
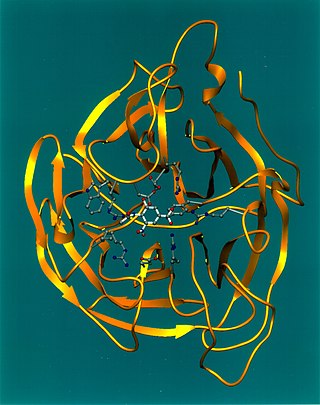
Exo-α-sialidase is a glycoside hydrolase that cleaves the glycosidic linkages of neuraminic acids:
Virus-like particles (VLPs) are molecules that closely resemble viruses, but are non-infectious because they contain no viral genetic material. They can be naturally occurring or synthesized through the individual expression of viral structural proteins, which can then self assemble into the virus-like structure. Combinations of structural capsid proteins from different viruses can be used to create recombinant VLPs. Both in-vivo assembly and in-vitro assembly have been successfully shown to form virus-like particles. VLPs derived from the Hepatitis B virus (HBV) and composed of the small HBV derived surface antigen (HBsAg) were described in 1968 from patient sera. VLPs have been produced from components of a wide variety of virus families including Parvoviridae, Retroviridae, Flaviviridae, Paramyxoviridae and bacteriophages. VLPs can be produced in multiple cell culture systems including bacteria, mammalian cell lines, insect cell lines, yeast and plant cells.

Sir Gregory Paul Winter is a Nobel Prize-winning English molecular biologist best known for his work on the therapeutic use of monoclonal antibodies. His research career has been based almost entirely at the MRC Laboratory of Molecular Biology and the MRC Centre for Protein Engineering, in Cambridge, England.
Neuraminidase inhibitors (NAIs) are a class of drugs which block the neuraminidase enzyme. They are a commonly used antiviral drug type against influenza. Viral neuraminidases are essential for influenza reproduction, facilitating viral budding from the host cell. Oseltamivir (Tamiflu), zanamivir (Relenza), laninamivir (Inavir), and peramivir belong to this class. Unlike the M2 inhibitors, which work only against the influenza A virus, NAIs act against both influenza A and influenza B.

In structural biology, a beta-propeller (β-propeller) is a type of all-β protein architecture characterized by 4 to 8 highly symmetrical blade-shaped beta sheets arranged toroidally around a central axis. Together the beta-sheets form a funnel-like active site.

In virology, a spike protein or peplomer protein is a protein that forms a large structure known as a spike or peplomer projecting from the surface of an enveloped virus. The proteins are usually glycoproteins that form dimers or trimers.

Viral neuraminidase is a type of neuraminidase found on the surface of influenza viruses that enables the virus to be released from the host cell. Neuraminidases are enzymes that cleave sialic acid groups from glycoproteins. Viral neuraminidase was discovered by Alfred Gottschalk at the Walter and Eliza Hall Institute in 1957. Neuraminidase inhibitors are antiviral agents that inhibit influenza viral neuraminidase activity and are of major importance in the control of influenza.

Hemagglutinins are homotrimeric glycoproteins present on the protein capsids of viruses in the Paramyxoviridae and Orthomyxoviridae families. Hemagglutinins are responsible for binding to receptors, sialic acid residues, on host cell membranes to initiate virus docking and infection.
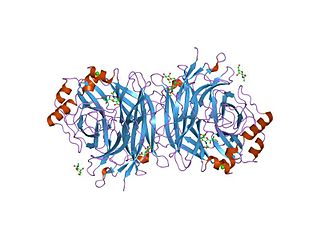
Hemagglutinin-neuraminidase refers to a single viral protein that has both hemagglutinin and (endo) neuraminidase EC 3.2.1.18 activity. This is in contrast to the proteins found in influenza, where both functions exist but in two separate proteins. Its neuraminidase domain has the CAZy designation glycoside hydrolase family 83 (GH83).
A heavy-chain antibody is an antibody which consists only of two heavy chains and lacks the two light chains usually found in antibodies.

Peter Palese is a United States microbiologist, researcher, inventor and the Horace W. Goldsmith Professor in the Department of Microbiology at the Icahn School of Medicine at Mount Sinai in New York City, and an expert in the field of RNA viruses.

George Keble Hirst, M.D. was an American virologist and science administrator who was among the first to study the molecular biology and genetics of animal viruses, especially influenza virus. He directed the Public Health Research Institute in New York City (1956–1981), and was also the founding editor-in-chief of Virology, the first English-language journal to focus on viruses. He is particularly known for inventing the hemagglutination assay, a simple method for quantifying viruses, and adapting it into the hemagglutination inhibition assay, which measures virus-specific antibodies in serum. He was the first to discover that viruses can contain enzymes, and the first to propose that virus genomes can consist of discontinuous segments. The New York Times described him as "a pioneer in molecular virology."
Neuraminidase inhibitors inhibit enzymatic activity of the enzyme neuraminidase (sialidase). These type of inhibitors have been introduced as anti-influenza drugs as they prevent the virus from exiting infected cells and thus stop further spreading of the virus. Neuraminidase inhibitors for human neuraminidase (hNEU) have the potential to be useful drugs as the enzyme plays a role in several signaling pathways in cells and is implicated in diseases such as diabetes and cancer.
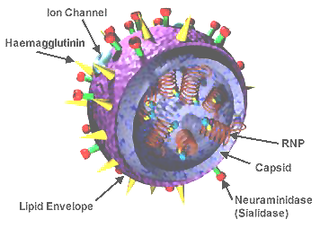
A universal flu vaccine would be a flu vaccine effective against all human-adapted strains of influenza A and influenza B regardless of the virus sub type, or any antigenic drift or antigenic shift. Hence it should not require modification from year to year in order to keep up with changes in the influenza virus. As of 2024 no universal flu vaccine had been successfully developed, however several candidate vaccines were in development, with some undergoing early stage clinical trial.
Gary J. Nabel is an American virologist and immunologist who is President and chief executive officer of ModeX Therapeutics in Natick, Massachusetts. He was the founding director of Vaccine Research Center at the National Institute of Allergy and Infectious Diseases.














