
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.

Electron ionization is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of the first ionization techniques developed for mass spectrometry. However, this method is still a popular ionization technique. This technique is considered a hard ionization method, since it uses highly energetic electrons to produce ions. This leads to extensive fragmentation, which can be helpful for structure determination of unknown compounds. EI is the most useful for organic compounds which have a molecular weight below 600 amu. Also, several other thermally stable and volatile compounds in solid, liquid and gas states can be detected with the use of this technique when coupled with various separation methods.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.

Gas chromatography–mass spectrometry (GC–MS) is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC–MS include drug detection, fire investigation, environmental analysis, explosives investigation, food and flavor analysis, and identification of unknown samples, including that of material samples obtained from planet Mars during probe missions as early as the 1970s. GC–MS can also be used in airport security to detect substances in luggage or on human beings. Additionally, it can identify trace elements in materials that were previously thought to have disintegrated beyond identification. Like liquid chromatography–mass spectrometry, it allows analysis and detection even of tiny amounts of a substance.

Metabolomics is the scientific study of chemical processes involving metabolites, the small molecule substrates, intermediates, and products of cell metabolism. Specifically, metabolomics is the "systematic study of the unique chemical fingerprints that specific cellular processes leave behind", the study of their small-molecule metabolite profiles. The metabolome represents the complete set of metabolites in a biological cell, tissue, organ, or organism, which are the end products of cellular processes. Messenger RNA (mRNA), gene expression data, and proteomic analyses reveal the set of gene products being produced in the cell, data that represents one aspect of cellular function. Conversely, metabolic profiling can give an instantaneous snapshot of the physiology of that cell, and thus, metabolomics provides a direct "functional readout of the physiological state" of an organism. There are indeed quantifiable correlations between the metabolome and the other cellular ensembles, which can be used to predict metabolite abundances in biological samples from, for example mRNA abundances. One of the ultimate challenges of systems biology is to integrate metabolomics with all other -omics information to provide a better understanding of cellular biology.

Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules are ionized by electron ionization to form reagent ions, which subsequently react with analyte molecules in the gas phase to create analyte ions for analysis by mass spectrometry. Negative chemical ionization (NCI), charge-exchange chemical ionization, atmospheric-pressure chemical ionization (APCI) and atmospheric pressure photoionization (APPI) are some of the common variants of the technique. CI mass spectrometry finds general application in the identification, structure elucidation and quantitation of organic compounds as well as some utility in biochemical analysis. Samples to be analyzed must be in vapour form, or else, must be vapourized before introduction into the source.

In mass spectrometry, matrix-assisted laser desorption/ionization (MALDI) is an ionization technique that uses a laser energy-absorbing matrix to create ions from large molecules with minimal fragmentation. It has been applied to the analysis of biomolecules and various organic molecules, which tend to be fragile and fragment when ionized by more conventional ionization methods. It is similar in character to electrospray ionization (ESI) in that both techniques are relatively soft ways of obtaining ions of large molecules in the gas phase, though MALDI typically produces far fewer multi-charged ions.

Liquid chromatography–mass spectrometry (LC–MS) is an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography with the mass analysis capabilities of mass spectrometry (MS). Coupled chromatography – MS systems are popular in chemical analysis because the individual capabilities of each technique are enhanced synergistically. While liquid chromatography separates mixtures with multiple components, mass spectrometry provides spectral information that may help to identify each separated component. MS is not only sensitive, but provides selective detection, relieving the need for complete chromatographic separation. LC–MS is also appropriate for metabolomics because of its good coverage of a wide range of chemicals. This tandem technique can be used to analyze biochemical, organic, and inorganic compounds commonly found in complex samples of environmental and biological origin. Therefore, LC–MS may be applied in a wide range of sectors including biotechnology, environment monitoring, food processing, and pharmaceutical, agrochemical, and cosmetic industries. Since the early 2000s, LC–MS has also begun to be used in clinical applications.

An electron capture detector (ECD) is a device for detecting atoms and molecules in a gas through the attachment of electrons via electron capture ionization. The device was invented in 1957 by James Lovelock and is used in gas chromatography to detect trace amounts of chemical compounds in a sample.

Atmospheric pressure chemical ionization (APCI) is an ionization method used in mass spectrometry which utilizes gas-phase ion-molecule reactions at atmospheric pressure (105 Pa), commonly coupled with high-performance liquid chromatography (HPLC). APCI is a soft ionization method similar to chemical ionization where primary ions are produced on a solvent spray. The main usage of APCI is for polar and relatively less polar thermally stable compounds with molecular weight less than 1500 Da. The application of APCI with HPLC has gained a large popularity in trace analysis detection such as steroids, pesticides and also in pharmacology for drug metabolites.
A mass chromatogram is a representation of mass spectrometry data as a chromatogram, where the x-axis represents time and the y-axis represents signal intensity. The source data contains mass information; however, it is not graphically represented in a mass chromatogram in favor of visualizing signal intensity versus time. The most common use of this data representation is when mass spectrometry is used in conjunction with some form of chromatography, such as in liquid chromatography–mass spectrometry or gas chromatography–mass spectrometry. In this case, the x-axis represents retention time, analogous to any other chromatogram. The y-axis represents signal intensity or relative signal intensity. There are many different types of metrics that this intensity may represent, depending on what information is extracted from each mass spectrum.
In mass spectrometry, direct analysis in real time (DART) is an ion source that produces electronically or vibronically excited-state species from gases such as helium, argon, or nitrogen that ionize atmospheric molecules or dopant molecules. The ions generated from atmospheric or dopant molecules undergo ion-molecule reactions with the sample molecules to produce analyte ions. Analytes with low ionization energy may be ionized directly. The DART ionization process can produce positive or negative ions depending on the potential applied to the exit electrode.

Field desorption (FD) is a method of ion formation used in mass spectrometry (MS) in which a high-potential electric field is applied to an emitter with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers" have formed. This results in a high electric field which can result in ionization of gaseous molecules of the analyte. Mass spectra produced by FD have little or no fragmentation because FD is a soft ionization method. They are dominated by molecular radical cations M+. and less often, protonated molecules . The technique was first reported by Beckey in 1969. It is also the first ionization method to ionize nonvolatile and thermally labile compounds. One major difference of FD with other ionization methods is that it does not need a primary beam to bombard a sample.

Desorption electrospray ionization (DESI) is an ambient ionization technique that can be coupled to mass spectrometry (MS) for chemical analysis of samples at atmospheric conditions. Coupled ionization sources-MS systems are popular in chemical analysis because the individual capabilities of various sources combined with different MS systems allow for chemical determinations of samples. DESI employs a fast-moving charged solvent stream, at an angle relative to the sample surface, to extract analytes from the surfaces and propel the secondary ions toward the mass analyzer. This tandem technique can be used to analyze forensics analyses, pharmaceuticals, plant tissues, fruits, intact biological tissues, enzyme-substrate complexes, metabolites and polymers. Therefore, DESI-MS may be applied in a wide variety of sectors including food and drug administration, pharmaceuticals, environmental monitoring, and biotechnology.
Sample preparation for mass spectrometry is used for the optimization of a sample for analysis in a mass spectrometer (MS). Each ionization method has certain factors that must be considered for that method to be successful, such as volume, concentration, sample phase, and composition of the analyte solution. Quite possibly the most important consideration in sample preparation is knowing what phase the sample must be in for analysis to be successful. In some cases the analyte itself must be purified before entering the ion source. In other situations, the matrix, or everything in the solution surrounding the analyte, is the most important factor to consider and adjust. Often, sample preparation itself for mass spectrometry can be avoided by coupling mass spectrometry to a chromatography method, or some other form of separation before entering the mass spectrometer. In some cases, the analyte itself must be adjusted so that analysis is possible, such as in protein mass spectrometry, where usually the protein of interest is cleaved into peptides before analysis, either by in-gel digestion or by proteolysis in solution.

Ion mobility spectrometry–mass spectrometry (IMS-MS) is an analytical chemistry method that separates gas phase ions based on their interaction with a collision gas and their masses. In the first step, the ions are separated according to their mobility through a buffer gas on a millisecond timescale using an ion mobility spectrometer. The separated ions are then introduced into a mass analyzer in a second step where their mass-to-charge ratios can be determined on a microsecond timescale. The effective separation of analytes achieved with this method makes it widely applicable in the analysis of complex samples such as in proteomics and metabolomics.

Atmospheric pressure photoionization (APPI) is a soft ionization method used in mass spectrometry (MS) usually coupled to liquid chromatography (LC). Molecules are ionized using a vacuum ultraviolet (VUV) light source operating at atmospheric pressure, either by direct absorption followed by electron ejection or through ionization of a dopant molecule that leads to chemical ionization of target molecules. The sample is usually a solvent spray that is vaporized by nebulization and heat. The benefit of APPI is that it ionizes molecules across a broad range of polarity and is particularly useful for ionization of low polarity molecules for which other popular ionization methods such as electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) are less suitable. It is also less prone to ion suppression and matrix effects compared to ESI and APCI and typically has a wide linear dynamic range. The application of APPI with LC/MS is commonly used for analysis of petroleum compounds, pesticides, steroids, and drug metabolites lacking polar functional groups and is being extensively deployed for ambient ionization particularly for explosives detection in security applications.

Desorption/ionization on silicon (DIOS) is a soft laser desorption method used to generate gas-phase ions for mass spectrometry analysis. DIOS is considered the first surface-based surface-assisted laser desorption/ionization (SALDI-MS) approach. Prior approaches were accomplished using nanoparticles in a matrix of glycerol, while DIOS is a matrix-free technique in which a sample is deposited on a nanostructured surface and the sample desorbed directly from the nanostructured surface through the adsorption of laser light energy. DIOS has been used to analyze organic molecules, metabolites, biomolecules and peptides, and, ultimately, to image tissues and cells.
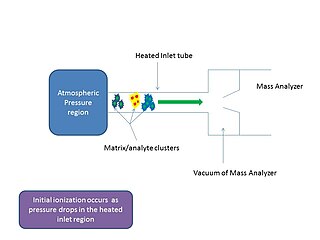
In mass spectrometry, matrix-assisted ionization is a low fragmentation (soft) ionization technique which involves the transfer of particles of the analyte and matrix sample from atmospheric pressure (AP) to the heated inlet tube connecting the AP region to the vacuum of the mass analyzer.

















