
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species replaces a functional group within another electron-deficient molecule. The molecule that contains the electrophile and the leaving functional group is called the substrate.
An allylic rearrangement or allylic shift is an organic chemical reaction in which reaction at a center vicinal to a double bond causes the double bond to shift to an adjacent pair of atoms:

Prenol, or 3-methyl-2-buten-1-ol, is a natural alcohol. It is one of the most simple terpenoids. It is a clear colorless oil that is reasonably soluble in water and miscible with most common organic solvents. It has a fruity odor and is used occasionally in perfumery.

The Wharton olefin synthesis or the Wharton reaction is a chemical reaction that involves the reduction of α,β-epoxy ketones using hydrazine to give allylic alcohols. This reaction, introduced in 1961 by P. S. Wharton, is an extension of the Wolff–Kishner reduction. The general features of this synthesis are: 1) the epoxidation of α,β-unsaturated ketones is achieved usually in basic conditions using hydrogen peroxide solution in high yield; 2) the epoxy ketone is treated with 2–3 equivalents of a hydrazine hydrate in presence of substoichiometric amounts of acetic acid. This reaction occurs rapidly at room temperature with the evolution of nitrogen and the formation of an allylic alcohol. It can be used to synthesize carenol compounds. Wharton's initial procedure has been improved.

Isoamyl alcohol is a colorless liquid with the formula C
5H
12O, specifically (H3C–)2CH–CH2–CH2–OH. It is one of several isomers of amyl alcohol (pentanol). It is also known as isopentyl alcohol, isopentanol, or (in the IUPAC recommended nomenclature) 3-methyl-butan-1-ol. An obsolete name for it was isobutyl carbinol.
In chemistry, a reaction intermediate, or intermediate, is a molecular entity arising within the sequence of a stepwise chemical reaction. It is formed as the reaction product of an elementary step, from the reactants and/or preceding intermediates, but is consumed in a later step. It does not appear in the chemical equation for the overall reaction.
The Favorskii reaction is an organic chemistry reaction between an alkyne and a carbonyl group, under basic conditions. The reaction was discovered in the early 1900s by the Russian chemist Alexei Yevgrafovich Favorskii.

Methyl acrylate is an organic compound, more accurately the methyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced to make acrylate fiber, which is used to weave synthetic carpets. It is also a reagent in the synthesis of various pharmaceutical intermediates. Owing to the tendency of methyl acrylate to polymerize, samples typically contain an inhibitor such as hydroquinone.

The Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis.
In organic chemistry, the Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is a cyclization reaction with remote C–H functionalization. In the reaction, thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid (concentrated sulfuric acid or concentrated CF3CO2H) generates a nitrogen radical intermediate. The radical then abstracts an intramolecular hydrogen atom to give a cyclic amine 2 (pyrrolidine or, in some cases, piperidine).
The Fukuyama coupling is a coupling reaction taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. The reaction product is a ketone. This reaction was discovered by Tohru Fukuyama et al. in 1998.

Isoprenol, also known as 3-methylbut-3-en-1-ol, is a hemiterpene alcohol. It is produced industrially as an intermediate to 3-methylbut-2-en-1-ol (prenol): global production in 2001 can be estimated as 6–13 thousand tons.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.
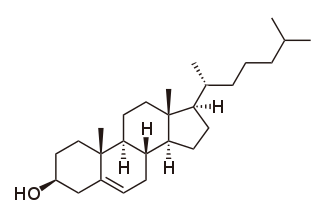
Cholesterol total synthesis in chemistry describes the total synthesis of the complex biomolecule cholesterol and is considered a great scientific achievement. The research group of Robert Robinson with John Cornforth published their synthesis in 1951 and that of Robert Burns Woodward with Franz Sondheimer in 1952. Both groups competed for the first publication since 1950 with Robinson having started in 1932 and Woodward in 1949. According to historian Greg Mulheirn the Robinson effort was hampered by his micromanagement style of leadership and the Woodward effort was greatly facilitated by his good relationships with chemical industry. Around 1949 steroids like cortisone were produced from natural resources but expensive. Chemical companies Merck & Co. and Monsanto saw commercial opportunities for steroid synthesis and not only funded Woodward but also provided him with large quantities of certain chemical intermediates from pilot plants. Hard work also helped the Woodward effort: one of the intermediate compounds was named Christmasterone as it was synthesized on Christmas Day 1950 by Sondheimer.
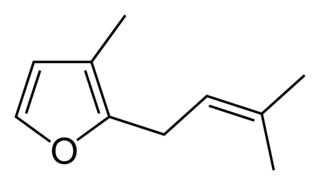
Rosefuran (3-methyl-2-prenylfuran) is an organic compound, classified as a terpenoid. It is a minor constituent of the aroma of the rose. Rosefuran is a 2,3-disubstituted furan. It has an odor threshold of 200 ppb and constitutes 0.16% of Bulgarian rose oil. Rosefuran has been established as a female sex pheromone of an acarid mite, Caloglyphus sp. Concentrations of less than 100 ng of synthetic rosefuran caused sexual excitation in males of the species.
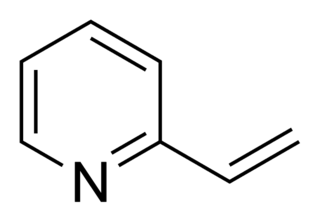
2-Vinylpyridine is an organic compound with the formula CH2CHC5H4N. It is a derivative of pyridine with a vinyl group in the 2-position, next to the nitrogen. It is a colorless liquid, although samples are often brown. It is used industrially as a precursor to specialty polymers and as an intermediate in the chemical, pharmaceutical, dye, and photo industries. Vinylpyridine is sensitive to polymerization. It may be stabilized with a polymerisation inhibitor such as tert-butylcatechol. Owing to its tendency to polymerize, samples are typically refrigerated.
The Danheiser benzannulation is a chemical reaction used in organic chemistry to generate highly substituted phenols in a single step. It is named after Rick L. Danheiser who developed the reaction.
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne is added to a carbonyl group to form an α-alkynyl alcohol.














